Early Microbes Modify Immune System Development and Metabolic Homeostasis-The "Restaurant" Hypothesis Revisited
- PMID: 29326657
- PMCID: PMC5733336
- DOI: 10.3389/fendo.2017.00349
Early Microbes Modify Immune System Development and Metabolic Homeostasis-The "Restaurant" Hypothesis Revisited
Abstract
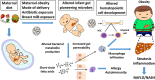
The developing infant gut microbiome affects metabolism, maturation of the gastrointestinal tract, immune system function, and brain development. Initial seeding of the neonatal microbiota occurs through maternal and environmental contact. Maternal diet, antibiotic use, and cesarean section alter the offspring microbiota composition, at least temporarily. Nutrients are thought to regulate initial perinatal microbial colonization, a paradigm known as the "Restaurant" hypothesis. This hypothesis proposes that early nutritional stresses alter both the initial colonizing bacteria and the development of signaling pathways controlled by microbial mediators. These stresses fine-tune the immune system and metabolic homeostasis in early life, potentially setting the stage for long-term metabolic and immune health. Dysbiosis, an imbalance or a maladaptation in the microbiota, can be caused by several factors including dietary alterations and antibiotics. Dysbiosis can alter biological processes in the gut and in tissues and organs throughout the body. Misregulated development and activity of both the innate and adaptive immune systems, driven by early dysbiosis, could have long-lasting pathologic consequences such as increased autoimmunity, increased adiposity, and non-alcoholic fatty liver disease (NAFLD). This review will focus on factors during pregnancy and the neonatal period that impact a neonate's gut microbiome, as well as the mechanisms and possible links from early infancy that can drive increased risk for diseases including obesity and NAFLD. The complex pathways that connect diet, the microbiota, immune system development, and metabolism, particularly in early life, present exciting new frontiers for biomedical research.
Keywords: innate immunity; microbiome; non-alcoholic fatty liver disease; pregnancy; proteobacteria.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

