Regulation of Fn14 Receptor and NF-κB Underlies Inflammation in Meniere's Disease
- PMID: 29326686
- PMCID: PMC5733484
- DOI: 10.3389/fimmu.2017.01739
Regulation of Fn14 Receptor and NF-κB Underlies Inflammation in Meniere's Disease
Abstract
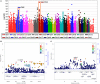
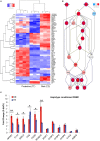
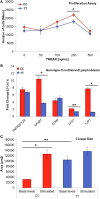
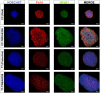
Meniere's disease (MD) is a rare disorder characterized by episodic vertigo, sensorineural hearing loss, tinnitus, and aural fullness. It is associated with a fluid imbalance between the secretion of endolymph in the cochlear duct and its reabsorption into the subarachnoid space, leading to an accumulation of endolymph in the inner ear. Epidemiological evidence, including familial aggregation, indicates a genetic contribution and a consistent association with autoimmune diseases (AD). We conducted a case-control study in two phases using an immune genotyping array in a total of 420 patients with bilateral MD and 1,630 controls. We have identified the first locus, at 6p21.33, suggesting an association with bilateral MD [meta-analysis leading signal rs4947296, OR = 2.089 (1.661-2.627); p = 1.39 × 10-09]. Gene expression profiles of homozygous genotype-selected peripheral blood mononuclear cells (PBMCs) demonstrated that this region is a trans-expression quantitative trait locus (eQTL) in PBMCs. Signaling analysis predicted several tumor necrosis factor-related pathways, the TWEAK/Fn14 pathway being the top candidate (p = 2.42 × 10-11). This pathway is involved in the modulation of inflammation in several human AD, including multiple sclerosis, systemic lupus erythematosus, or rheumatoid arthritis. In vitro studies with genotype-selected lymphoblastoid cells from patients with MD suggest that this trans-eQTL may regulate cellular proliferation in lymphoid cells through the TWEAK/Fn14 pathway by increasing the translation of NF-κB. Taken together; these findings suggest that the carriers of the risk genotype may develop an NF-κB-mediated inflammatory response in MD.
Keywords: Meniere’s disease; NF-κB signaling; NFKB1; TNFRSF12A; TWEAK/Fn14 pathway; sensorineural hearing loss; vertigo.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

