Targeting and Recognition of Toll-Like Receptors by Plant and Pathogen Lectins
- PMID: 29326706
- PMCID: PMC5741612
- DOI: 10.3389/fimmu.2017.01820
Targeting and Recognition of Toll-Like Receptors by Plant and Pathogen Lectins
Abstract
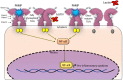
We have reported that some lectins act as agonists of toll-like receptors (TLRs) and have immunomodulatory properties. The plant lectin ArtinM, for example, interacts with N-glycans of TLR2, whereas other lectins of microbial origin interact with TLR2 and TLR4. Expression of the receptors on the surface of antigen-presenting cells exposes N-glycans that may be targeted by lectins of different structures, specificities, and origins. In vitro, these interactions trigger cell signaling that leads to NF-κB activation and production of the Th1 polarizing cytokine IL-12. In vivo, a same sequence of events follows the administration of an active lectin to mice infected with an intracellular pathogen, conferring resistance to the pathogen. The lectins of the human pathogens Toxoplasma gondii (TgMIC1 and TgMIC4) and Paracoccidioides brasiliensis (Paracoccin), by recognition and activation of TLR2 and TLR4, induce cell events and in vivo effects comparable to the promoted by the plant lectin ArtinM. In this article, we highlight these two distinct mechanisms for activating antigen-presenting cells. On the one hand, TLRs act as sensors for the presence of conventional pathogen-associated molecular patterns, such as microbial lipids. On the other hand, we showed that TLR-mediated cell activation might be triggered by an alternative way, in which lectins bind to TLRs N-glycans and stimulate cells to increase the expression of pro-inflammatory cytokines. This process may lead to the development of new pharmaceutical tools that promote protective immune responses directed against intracellular pathogens and tumors.
Keywords: N-glycosylation; carbohydrate recognition domain; innate immune response; lectins; toll-like receptors.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

