Extensive Basal Level Activation of Complement Mannose-Binding Lectin-Associated Serine Protease-3: Kinetic Modeling of Lectin Pathway Activation Provides Possible Mechanism
- PMID: 29326707
- PMCID: PMC5741598
- DOI: 10.3389/fimmu.2017.01821
Extensive Basal Level Activation of Complement Mannose-Binding Lectin-Associated Serine Protease-3: Kinetic Modeling of Lectin Pathway Activation Provides Possible Mechanism
Abstract
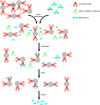
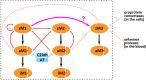
Serine proteases (SPs) are typically synthesized as precursors, termed proenzymes or zymogens, and the fully active form is produced via limited proteolysis by another protease or by autoactivation. The lectin pathway of the complement system is initiated by mannose-binding lectin (MBL)-associated SPs (MASP)-1, and MASP-2, which are known to be present as proenzymes in blood. The third SP of the lectin pathway, MASP-3, was recently shown to be the major activator, and the exclusive "resting blood" activator of profactor D, producing factor D, the initiator protease of the alternative pathway. Because only activated MASP-3 is capable of carrying out this cleavage, it was presumed that a significant fraction of MASP-3 must be present in the active form in resting blood. Here, we aimed to detect active MASP-3 in the blood by a more direct technique and to quantitate the active to zymogen ratio. First, MASPs were partially purified (enriched) from human plasma samples by affinity chromatography using immobilized MBL in the presence of inhibitors. Using this MASP pool, only the zymogen form of MASP-1 was detected by Western blot, whereas over 70% MASP-3 was in an activated form in the same samples. Furthermore, the active to zymogen ratio of MASP-3 showed little individual variation. It is enigmatic how MASP-3, which is not able to autoactivate, is present mostly as an active enzyme, whereas MASP-1, which has a potent autoactivation capability, is predominantly proenzymic in resting blood. In an attempt to explain this phenomenon, we modeled the basal level fluid-phase activation of lectin pathway proteases and their subsequent inactivation by C1 inhibitor and antithrombin using available and newly determined kinetic constants. The model can explain extensive MASP-3 activation only if we assume efficient intracomplex activation of MASP-3 by zymogen MASP-1. On the other hand, the model is in good agreement with the fact that MASP-1 and -2 are predominantly proenzymic and some of them is present in the form of inactive serpin-protease complexes. As an alternative hypothesis, MASP-3 activation by proprotein convertases is also discussed.
Keywords: autoactivation; complement; innate immunity; lectin pathway; proenzyme; reaction kinetics; serine protease.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

