Integrating Patient Perspectives into Personalized Medicine in Idiopathic Pulmonary Fibrosis
- PMID: 29326935
- PMCID: PMC5742327
- DOI: 10.3389/fmed.2017.00226
Integrating Patient Perspectives into Personalized Medicine in Idiopathic Pulmonary Fibrosis
Abstract
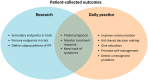
Idiopathic pulmonary fibrosis (IPF) is a progressive and ultimately fatal disease which has a major impact on patients' quality of life (QOL). Except for lung transplantation, there is no curative treatment option. Fortunately, two disease-modifying drugs that slow down disease decline were recently approved. Though this is a major step forward, these drugs do not halt or reverse the disease, nor convincingly improve health-related QOL. In daily practice, disease behavior and response to therapy greatly vary among patients. It is assumed that this is related to the multiple biological pathways and complex interactions between genetic, molecular, and environmental factors that are involved in the pathogenesis of IPF. Recently, research in IPF has therefore started to focus on developing targeted therapy through identifying genetic risk factors and biomarkers. In this rapidly evolving field of personalized medicine, patient factors such as lifestyle, comorbidities, preferences, and experiences with medication should not be overlooked. This review describes recent insights and methods on how to integrate patient perspectives into personalized medicine. Furthermore, it provides an overview of the most used patient-reported outcome measures in IPF, to facilitate choices for both researchers and clinicians when incorporating the patient voice in their research and care. To enhance truly personalized treatment in IPF, biology should be combined with patient perspectives.
Keywords: health-related quality of life; idiopathic pulmonary fibrosis; patient experiences; patient-reported outcomes; personalized medicine; personomics.
Figures
References
-
- Potter P. Hippocrates Vol VI. Diseases, Internal Affections. Cambridge, MA, London: Harvard University Press, William Heinemann LTD; (1988).
-
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med (2002) 165(2):277–304.10.1164/ajrccm.165.2.ats01 - DOI - PubMed
-
- The Voice of the Patient: A Series of Reports from the U.S. Food and Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative. Center for Drug Evaluation and Research (CDER) US Food and Drug Administration (FDA) (2015).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

