Comparing Neoadjuvant Nab-paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2-Negative Breast Cancer-The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial
- PMID: 29327055
- PMCID: PMC5885830
- DOI: 10.1001/jamaoncol.2017.4612
Comparing Neoadjuvant Nab-paclitaxel vs Paclitaxel Both Followed by Anthracycline Regimens in Women With ERBB2/HER2-Negative Breast Cancer-The Evaluating Treatment With Neoadjuvant Abraxane (ETNA) Trial: A Randomized Phase 3 Clinical Trial
Abstract
Importance: Studies of neoadjuvant chemotherapy regimens using anthracyclines followed by taxanes have reported a doubling of pathological complete remission (pCR) rates compared with anthracycline-based regimens alone. A reverse sequence did not reduce activity. Nab-paclitaxel is an albumin-bound nanoparticle of paclitaxel that allows for safe infusion without premedication, and its use led to a significantly higher rate of pCR in the GeparSepto trial.
Objective: To determine whether nab-paclitaxel improves the outcomes of early and locally advanced human epidermal growth factor receptor 2 (ERBB2/HER2)-negative breast cancer compared with paclitaxel when delivered in a neoadjuvant setting.
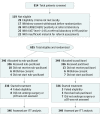
Design, setting, and participants: In this multicenter, open-label study, in collaboration with Grupo Español de Investigación en Cáncer de Mama (GEICAM) and Breast Cancer Research Center-Western Australia (BCRC-WA), patients with newly diagnosed and centrally confirmed ERBB2/HER2-negative breast cancer were recruited. Participants were randomly allocated to paclitaxel, 90 mg/m2 (349 patients), or nab-paclitaxel, 125 mg/m2 (346 patients). The 2 drugs were given on weeks 1, 2, and 3 followed by 1 week of rest for 4 cycles before 4 cycles of an anthracycline regimen per investigator choice.
Main outcomes and measures: The primary end point was the rate of pCR, defined as absence of invasive cells in the breast and axillary nodes (ie, ypT0/is ypN0) at the time of surgery. A secondary end point was to assess tolerability and safety of the 2 regimens.
Results: From May 2013 to March 2015, 814 patients were registered to the study; 695 patients met central confirmation eligibility and were randomly allocated to receive either paclitaxel (349), or nab-paclitaxel (346) (median age, 50 years; range, 25-79 years). The intention-to-treat analysis of the primary end point pCR revealed that the improved pCR rate after nab-paclitaxel (22.5%) was not statistically significant compared with paclitaxel (18.6%; odds ratio [OR], 0.77; 95% CI, 0.52-1.13; P = .19). Overall, 38 of 335 patients (11.3%) 11.3% of patients had at least 1 serious adverse event in the paclitaxel arm and 54 of 337 patient (16.0%) in the nab-paclitaxel arm. Peripheral neuropathy of grade 3 or higher occurred in 6 of 335 patients (1.8%) and in 15 of 337 (4.5%), respectively.
Conclusions and relevance: The improved rate of pCR after nab-paclitaxel was not statistically significant. The multivariate analysis revealed that tumor subtype (triple-negative vs luminal B-like) was the most significant factor (OR, 4.85; 95% CI, 3.28-7.18) influencing treatment outcome.
Trial registration: clinicaltrials.gov Identifier: NCT01822314.
Conflict of interest statement
Figures
Comment in
-
Neoadjuvant Systemic Therapy for Breast Cancer: Searching for More Effectively Curative Therapies.JAMA Oncol. 2018 Mar 1;4(3):293-295. doi: 10.1001/jamaoncol.2017.4651. JAMA Oncol. 2018. PMID: 29327056 No abstract available.
References
-
- Untch M, Jackisch C, Schneeweiss A, et al. ; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) Investigators . Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345-356. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. . Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172. - PubMed
-
- Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366(26):2438-2441. - PubMed
-
- Carey LA, Winer EP. I-SPY 2: toward more rapid progress in breast cancer treatment. N Engl J Med. 2016;375(1):83-84. - PubMed
-
- Mamounas EP, Bryant J, Lembersky B, et al. . Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23(16):3686-3696. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous