Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review
- PMID: 29327237
- PMCID: PMC7087223
- DOI: 10.1007/s00705-017-3700-y
Regional, age and respiratory-secretion-specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review
Abstract
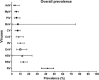
Despite increased understanding of how viral infection is involved in asthma exacerbations, it is less clear which viruses are involved and to what extent they contribute to asthma exacerbations. Here, we sought to determine the prevalence of different respiratory viruses during asthma exacerbations. Systematic computerized searches of the literature up to June 2017 without language limitation were performed. The primary focus was on the prevalence of respiratory viruses, including AdV (adenovirus), BoV (bocavirus), CoV (coronavirus), CMV (cytomegalovirus), EnV (enterovirus), HSV (herpes simplex virus), IfV (influenza virus), MpV (metapneumovirus), PiV (parainfluenzavirus), RV (rhinovirus) and RSV (respiratory syncytial virus) during asthma exacerbations. We also examined the prevalence of viral infection stratified by age, geographic region, type of respiratory secretion, and detection method. Sixty articles were included in the final analysis. During asthma exacerbations, the mean prevalence of AdV, BoV, CoV, CMV, EnV, HSV, IfV, MpV, PiV, RV and RSV was 3.8%, 6.9%, 8.4%, 7.2%, 10.1%, 12.3%, 10.0%, 5.3%, 5.6%, 42.1% and 13.6%, respectively. EnV, MPV, RV and RSV were more prevalent in children, whereas AdV, BoV, CoV, IfV and PiV were more frequently present in adults. RV was the major virus detected globally, except in Africa. RV could be detected in both the upper and lower airway. Polymerase chain reaction was the most sensitive method for detecting viral infection. Our findings indicate the need to develop prophylactic polyvalent or polyvirus (including RV, EnV, IfV and RSV) vaccines that produce herd immunity and reduce the healthcare burden associated with virus-induced asthma exacerbations.
Conflict of interest statement
Xue-yan Zheng declares that she had no conflict of interest. Yan-jun Xu declares that she had no conflict of interest. Li-feng Lin declares that he had no conflict of interest. Wei-jie Guan declares that he had no conflict of interest.
Figures

References
-
- GBD 2015 Chronic Respiratory Disease Collaborators Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, Bonini S, Bont L, Bossios A, Bousquet J, Braido F, Brusselle G, Canonica GW, Carlsen KH, Chanez P, Fokkens WJ, Garcia-Garcia M, Gjomarkaj M, Haahtela T, Holgate ST, Johnston SL, Konstantinou G, Kowalski M, Lewandowska-Polak A, Lødrup-Carlsen K, Mäkelä M, Malkusova I, Mullol J, Nieto A, Eller E, Ozdemir C, Panzner P, Popov T, Psarras S, Roumpedaki E, Rukhadze M, Stipic-Markovic A, Todo Bom A, Toskala E, van Cauwenberge P, van Drunen C, Watelet JB, Xatzipsalti M, Xepapadaki P, Zuberbier T. Viruses and bacteria in acute asthma exacerbations—a GA2LEN-DARE systematic review. Allergy. 2011;66:458–468. doi: 10.1111/j.1398-9995.2010.02505.x. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

