Strengthening standards, transparency, and collaboration to support medicine evaluation: Ten years of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP)
- PMID: 29327451
- PMCID: PMC5873428
- DOI: 10.1002/pds.4381
Strengthening standards, transparency, and collaboration to support medicine evaluation: Ten years of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP)
Figures
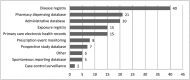
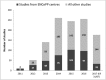
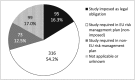

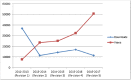
References
-
- Blake KV, de Vries CS, Arlett P, Kurz X, Fitt H. Increasing scientific standards, independence and transparency in post‐authorisation studies: the role of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Pharmacoepidemiol Drug Saf. 2012;21(7):690‐696. - PubMed
-
- Laporte JR. Fifty years of pharmacovigilance—medicines safety and public health. Pharmacoepidemiol Drug Saf. 2016;25(6):725‐732. - PubMed
-
- Arlett P, Sarac SB, Thomson A, et al. The European Medicines Agency's use of prioritised independent research for best evidence in regulatory action on diclofenac. Pharmacoepidemiol Drug Saf. 2014;23(4):431‐434. - PubMed
-
- Blake KV, Prilla S, Accadebled S, et al. European Medicines Agency review of post‐authorisation studies with implications for the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Pharmacoepidemiol Drug Saf. 2011;20(10):1021‐1029. - PubMed
-
- Persson I, Fitt H, Jekerle V. Model for ENCePP. European Medicines Agency, May 2007. http://www.encepp.eu/publications/documents/ENCePPConceptPaper_May07.pdf [last accessed on 12/10/2017]
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

