Natural and Artificial Strategies To Control the Conjugative Transmission of Plasmids
- PMID: 29327679
- PMCID: PMC11633558
- DOI: 10.1128/microbiolspec.MTBP-0015-2016
Natural and Artificial Strategies To Control the Conjugative Transmission of Plasmids
Abstract
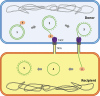
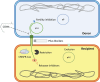
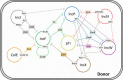
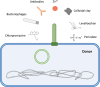
Conjugative plasmids are the main carriers of transmissible antibiotic resistance (AbR) genes. For that reason, strategies to control plasmid transmission have been proposed as potential solutions to prevent AbR dissemination. Natural mechanisms that bacteria employ as defense barriers against invading genomes, such as restriction-modification or CRISPR-Cas systems, could be exploited to control conjugation. Besides, conjugative plasmids themselves display mechanisms to minimize their associated burden or to compete with related or unrelated plasmids. Thus, FinOP systems, composed of FinO repressor protein and FinP antisense RNA, aid plasmids to regulate their own transfer; exclusion systems avoid conjugative transfer of related plasmids to the same recipient bacteria; and fertility inhibition systems block transmission of unrelated plasmids from the same donor cell. Artificial strategies have also been designed to control bacterial conjugation. For instance, intrabodies against R388 relaxase expressed in recipient cells inhibit plasmid R388 conjugative transfer; pIII protein of bacteriophage M13 inhibits plasmid F transmission by obstructing conjugative pili; and unsaturated fatty acids prevent transfer of clinically relevant plasmids in different hosts, promoting plasmid extinction in bacterial populations. Overall, a number of exogenous and endogenous factors have an effect on the sophisticated process of bacterial conjugation. This review puts them together in an effort to offer a wide picture and inform research to control plasmid transmission, focusing on Gram-negative bacteria.
Figures
Similar articles
-
Conjugation efficiency depends on intra and intercellular interactions between distinct plasmids: Plasmids promote the immigration of other plasmids but repress co-colonizing plasmids.Plasmid. 2017 Sep;93:6-16. doi: 10.1016/j.plasmid.2017.08.003. Epub 2017 Aug 24. Plasmid. 2017. PMID: 28842132
-
Towards a better understanding of antimicrobial resistance dissemination: what can be learnt from studying model conjugative plasmids?Mil Med Res. 2022 Jan 10;9(1):3. doi: 10.1186/s40779-021-00362-z. Mil Med Res. 2022. PMID: 35012680 Free PMC article. Review.
-
Cryptic environmental conjugative plasmid recruits a novel hybrid transposon resulting in a new plasmid with higher dispersion potential.mSphere. 2024 Jun 25;9(6):e0025224. doi: 10.1128/msphere.00252-24. Epub 2024 May 21. mSphere. 2024. PMID: 38771049 Free PMC article.
-
Detection of Horizontal Gene Transfer Mediated by Natural Conjugative Plasmids in E. coli.J Vis Exp. 2023 Mar 24;(193). doi: 10.3791/64523. J Vis Exp. 2023. PMID: 37036197
-
Regulation of bacterial conjugation: balancing opportunity with adversity.Future Microbiol. 2010 Jul;5(7):1057-71. doi: 10.2217/fmb.10.70. Future Microbiol. 2010. PMID: 20632805 Review.
Cited by
-
Assessing the Role of Bacterial Innate and Adaptive Immunity as Barriers to Conjugative Plasmids.Mol Biol Evol. 2024 Oct 4;41(10):msae207. doi: 10.1093/molbev/msae207. Mol Biol Evol. 2024. PMID: 39382385 Free PMC article.
-
Characterization of a mobilizable megaplasmid carrying multiple resistance genes from a clinical isolate of Pseudomonas aeruginosa.Front Microbiol. 2023 Nov 27;14:1293443. doi: 10.3389/fmicb.2023.1293443. eCollection 2023. Front Microbiol. 2023. PMID: 38088964 Free PMC article.
-
A Broad-Host-Range Plasmid Outbreak: Dynamics of IncL/M Plasmids Transferring Carbapenemase Genes.Antibiotics (Basel). 2022 Nov 17;11(11):1641. doi: 10.3390/antibiotics11111641. Antibiotics (Basel). 2022. PMID: 36421285 Free PMC article.
-
Soil Component: A Potential Factor Affecting the Occurrence and Spread of Antibiotic Resistance Genes.Antibiotics (Basel). 2023 Feb 4;12(2):333. doi: 10.3390/antibiotics12020333. Antibiotics (Basel). 2023. PMID: 36830244 Free PMC article. Review.
-
CRISPR-Cas systems are widespread accessory elements across bacterial and archaeal plasmids.Nucleic Acids Res. 2022 May 6;50(8):4315-4328. doi: 10.1093/nar/gkab859. Nucleic Acids Res. 2022. PMID: 34606604 Free PMC article.
References
-
- Fleming A. 1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. Influenzae. Br J Exp Pathol 10:226–236. [PubMed]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

