Blockade of the Complement C5a/C5aR1 Axis Impairs Lung Cancer Bone Metastasis by CXCL16-mediated Effects
- PMID: 29327939
- PMCID: PMC6835094
- DOI: 10.1164/rccm.201703-0660OC
Blockade of the Complement C5a/C5aR1 Axis Impairs Lung Cancer Bone Metastasis by CXCL16-mediated Effects
Abstract
Rationale: C5aR1 (CD88), a receptor for complement anaphylatoxin C5a, is a potent immune mediator. Its impact on malignant growth and dissemination of non-small cell lung cancer cells is poorly understood.
Objectives: To investigate the contribution of the C5a/C5aR1 axis to the malignant phenotype of non-small cell lung cancer cells, particularly in skeletal colonization, a preferential lung metastasis site.
Methods: Association between C5aR1 expression and clinical outcome was assessed in silico and validated by immunohistochemistry. Functional significance was evaluated by lentiviral gene silencing and ligand l-aptamer inhibition in in vivo models of lung cancer bone metastasis. In vitro functional assays for signaling, migration, invasion, metalloprotease activity, and osteoclastogenesis were also performed.
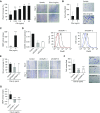
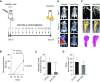
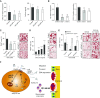
Measurements and main results: High levels of C5aR1 in human lung tumors were significantly associated with shorter recurrence-free survival, overall survival, and bone metastasis. Silencing of C5aR1 in lung cancer cells led to a substantial reduction in skeletal metastatic burden and osteolysis in in vivo models. Furthermore, metalloproteolytic, migratory, and invasive tumor cell activities were modulated in vitro by C5aR1 stimulation or gene silencing. l-Aptamer blockade or C5aR1 silencing significantly reduced the osseous metastatic activity of lung cancer cells in vivo. This effect was associated with decreased osteoclastogenic activity in vitro and was rescued by the exogenous addition of the chemokine CXCL16.
Conclusions: Disruption of C5aR1 signaling in lung cancer cells abrogates their tumor-associated osteoclastogenic activity, impairing osseous colonization. This study unveils the role played by the C5a/C5aR1 axis in lung cancer dissemination and supports its potential use as a novel therapeutic target.
Keywords: anaphylatoxin; aptamer; chemokine; complement; osteoclastogensesis.
Figures



Comment in
-
Complementing Lung Cancer: How Tumor Cells Co-opt the Host Complement System to Reach Bone.Am J Respir Crit Care Med. 2018 May 1;197(9):1101-1103. doi: 10.1164/rccm.201801-0032ED. Am J Respir Crit Care Med. 2018. PMID: 29365275 No abstract available.
References
-
- Croker DE, Monk PN, Halai R, Kaeslin G, Schofield Z, Wu MC, et al. Discovery of functionally selective C5aR2 ligands: novel modulators of C5a signalling. Immunol Cell Biol. 2016;94:787–795. - PubMed
-
- Ajona D, Castaño Z, Garayoa M, Zudaire E, Pajares MJ, Martinez A, et al. Expression of complement factor H by lung cancer cells: effects on the activation of the alternative pathway of complement. Cancer Res. 2004;64:6310–6318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

