Infection Is Not Required for Mucoinflammatory Lung Disease in CFTR-Knockout Ferrets
- PMID: 29327941
- PMCID: PMC5955060
- DOI: 10.1164/rccm.201708-1616OC
Infection Is Not Required for Mucoinflammatory Lung Disease in CFTR-Knockout Ferrets
Abstract
Rationale: Classical interpretation of cystic fibrosis (CF) lung disease pathogenesis suggests that infection initiates disease progression, leading to an exuberant inflammatory response, excessive mucus, and ultimately bronchiectasis. Although symptomatic antibiotic treatment controls lung infections early in disease, lifelong bacterial residence typically ensues. Processes that control the establishment of persistent bacteria in the CF lung, and the contribution of noninfectious components to disease pathogenesis, are poorly understood.
Objectives: To evaluate whether continuous antibiotic therapy protects the CF lung from disease using a ferret model that rapidly acquires lethal bacterial lung infections in the absence of antibiotics.
Methods: CFTR (cystic fibrosis transmembrane conductance regulator)-knockout ferrets were treated with three antibiotics from birth to several years of age and lung disease was followed by quantitative computed tomography, BAL, and histopathology. Lung disease was compared with CFTR-knockout ferrets treated symptomatically with antibiotics.
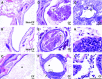
Measurements and main results: Bronchiectasis was quantified from computed tomography images. BAL was evaluated for cellular differential and features of inflammatory cellular activation, bacteria, fungi, and quantitative proteomics. Semiquantitative histopathology was compared across experimental groups. We demonstrate that lifelong antibiotics can protect the CF ferret lung from infections for several years. Surprisingly, CF animals still developed hallmarks of structural bronchiectasis, neutrophil-mediated inflammation, and mucus accumulation, despite the lack of infection. Quantitative proteomics of BAL from CF and non-CF pairs demonstrated a mucoinflammatory signature in the CF lung dominated by Muc5B and neutrophil chemoattractants and products.
Conclusions: These findings implicate mucoinflammatory processes in the CF lung as pathogenic in the absence of clinically apparent bacterial and fungal infections.
Keywords: airway obstruction; cystic fibrosis; ferret; inflammation; mucus.
Figures





Comment in
-
Ferreting Out the Role of Infection in Cystic Fibrosis Lung Disease.Am J Respir Crit Care Med. 2018 May 15;197(10):1243-1244. doi: 10.1164/rccm.201801-0053ED. Am J Respir Crit Care Med. 2018. PMID: 29357264 No abstract available.
-
How to live a long and healthy life with cystic fibrosis: Lessons from the CF ferret.J Cyst Fibros. 2019 Jan;18(1):8-9. doi: 10.1016/j.jcf.2018.10.005. Epub 2018 Oct 23. J Cyst Fibros. 2019. PMID: 30361142 No abstract available.
References
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR.Cystic fibrosis Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B.editorsThe metabolic and molecular basis of inherited disease 8th edNew York: McGraw-Hill: 20015121–5189.
-
- Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, et al. Innate immunity in cystic fibrosis lung disease. 2012;11:363–382. - PubMed
-
- Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. 1995;151:1075–1082. - PubMed
-
- Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. 2009;180:146–152. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

