Control of directionality in lambda site specific recombination
- PMID: 2932798
- PMCID: PMC1978455
- DOI: 10.1126/science.2932798
Control of directionality in lambda site specific recombination
Abstract
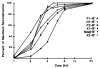
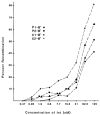
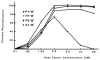
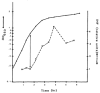
The simple relation between the substrates and products of site-specific recombination raises questions about the control of directionality often observed in this class of DNA transactions. For bacteriophage lambda, viral integration and excision proceed by discrete pathways, and DNA substrates with the intrinsic property of recombining in only one direction can be constructed. These pathways display an asymmetric reliance on a complex array of protein binding sites, and they respond differently to changes in the concentrations of the relevant proteins. The Escherichia coli protein integration host factor (IHF) differentially affects integrative and excisive recombination, thereby influencing directionality. A four- to eightfold increase in intracellular IHF coincides with the transition from exponential to stationary phase; this provides a mechanism for growth phase-dependent regulation of recombination that makes the cellular physiology an intrinsic part of the recombination reaction.
Figures



References
-
- Weisberg RA, Landy A. In: Lambda II. Hendrix R, Roberts J, Stahl F, Weisberg R, editors. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1983. p. 211.
-
- Nash HA. Annu Rev Genet. 1981;15:143. - PubMed
-
- Mizuuchi K, et al. Cold Spring Harbor Symp Quant Biol. 1981;45:429. - PubMed
-
- Bauer CE, Gardner JF, Gumport RI. J Mol Biol. 1985;181:187. - PubMed
-
- Holliday R. Genet Res. 1974;5:282.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

