Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway
- PMID: 29328487
- PMCID: PMC5802029
- DOI: 10.3892/or.2018.6198
Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway
Abstract
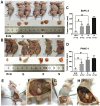
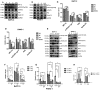
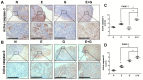
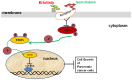
Compared to single gemcitabine treatment, the combination of gemcitabine and erlotinib has shown effective response in patients with locally advanced or metastatic pancreatic cancer. However, the combination therapy has not proven effective in patients with pancreatic cancer after R0 or R1 resection. In the present study, a nude mice model of orthotopic xenotransplantation after tumor resection was established using pancreatic cancer cell lines, BxPC-3 and PANC‑1. Mice were divided in four groups (each with n=12) and were treated as follows: the control group received a placebo via intraperitoneal injection (i.p.), while the other three groups were treated with gemcitabine (50 mg/kg i.p., twice a week), erlotinib (50 mg/kg oral gavage, once every three days), and combined treatment of gemcitabine and erlotinib, respectively. The treatment lasted for 21 days, after which all mice were sacrificed and tumors were examined ex vivo. We determined that the combination of gemcitabine and erlotinib inhibited recurrent tumor growth and induced apoptosis in vivo by downregulating phosphorylation levels of JAKs and STATs, which in turn downregulated the downstream proteins HIF‑1α and cyclin D1, and upregulated caspase‑9 and caspase‑3 expression. To sum up, the combination of gemcitabine with erlotinib was effective in treating patients with pancreatic cancer after R0 or R1 resection.
Figures


References
-
- Wang Y, Hu GF, Zhang QQ, Tang N, Guo J, Liu LY, Han X, Wang X, Wang ZH. Efficacy and safety of gemcitabine plus erlotinib for locally advanced or metastatic pancreatic cancer: A systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1961–1972. doi: 10.2147/DDDT.S105442. - DOI - PMC - PubMed
-
- Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, et al. ESMO Guidelines Committee Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. doi: 10.1093/annonc/mdv295. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

