Failure of monotherapy in clinical practice in patients with type 2 diabetes: The Korean National Diabetes Program
- PMID: 29328551
- PMCID: PMC6123024
- DOI: 10.1111/jdi.12801
Failure of monotherapy in clinical practice in patients with type 2 diabetes: The Korean National Diabetes Program
Abstract
Aims/introduction: We investigated the failure of monotherapy in patients with type 2 diabetes mellitus in real practice settings.
Materials and methods: The Korean National Diabetes Program was a prospective, multicenter observational cohort study of type 2 diabetes mellitus patients in Korea. Of the 3,950 patients enrolled in the study, we studied 998 who were continuously maintained on monotherapy for at least 90 days at six participating centers. To balance the baseline characteristics of patients in each group, we used propensity matching at a 1:1 ratio (metformin vs sulfonylureas) and 4:1 ratio (metformin vs meglitinides and metformin vs alpha-glucosidase inhibitors [aGIs]). The hazard ratios (HRs) of treatments (compared with metformin) were determined by Cox's proportional hazards regression modeling.
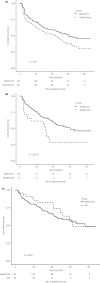
Results: The median follow-up time was 56 months, and monotherapy failed in 45% of all patients. The annual incidences of failure were 15.6%, 21.3%, 27% and 9.6% in the metformin, sulfonylurea, meglitinide and aGI groups. Compared with metformin, sulfonylureas and meglitinides were associated with higher risks of monotherapy failure (HR 1.39, 95% confidence interval [CI] 1.08-1.80; HR 1.92, 95% CI 1.13-3.27), and aGIs with risks similar to that of metformin (HR 0.80, 95% CI 0.44-1.45). When analyzed by failure type, sulfonylureas, meglitinides and aGIs were associated with a higher risk of a switch to other agents (HR 4.43, 95% CI 2.14-9.17; HR 18.80, 95% CI 6.21-56.93; HR 4.25, 95% CI 1.49-12.13), and aGIs with a lower risk of prescription of add-on second agents (HR 0.16, 95% CI 0.04-0.64).
Conclusions: Metformin was associated with a lower failure risk than were sulfonylureas and meglitinides, but a comparable aGI failure rate.
Keywords: Cohort study; Monotherapy failure; Type 2 diabetes.
© 2018 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures
References
-
- American Diabetes Association . 6. Glycemic targets. Diabetes Care 2017; 40(Suppl 1): S48–S56. - PubMed
-
- American Diabetes Association . 8. Pharmacologic approaches to glycemic treatment. Diabetes Care 2017; 40(Suppl 1): S64–S74. - PubMed
-
- U.K. Prospective Diabetes Study Group . U.K. Prospective diabetes study 16. Overview of 6 years' therapy of type II diabetes: a progressive disease. Diabetes 1995; 44: 1249–1258. - PubMed
-
- Kahn SE. Clinical review 135: the importance of beta‐cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metabol 2001; 86: 4047–4058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical