DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information
- PMID: 29328784
- PMCID: PMC6080696
- DOI: 10.1146/annurev-biochem-062917-012550
DNA-Encoded Chemical Libraries: A Selection System Based on Endowing Organic Compounds with Amplifiable Information
Abstract
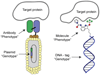
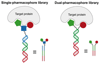
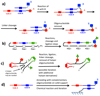
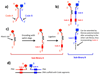
The discovery of organic ligands that bind specifically to proteins is a central problem in chemistry, biology, and the biomedical sciences. The encoding of individual organic molecules with distinctive DNA tags, serving as amplifiable identification bar codes, allows the construction and screening of combinatorial libraries of unprecedented size, thus facilitating the discovery of ligands to many different protein targets. Fundamentally, one links powers of genetics and chemical synthesis. After the initial description of DNA-encoded chemical libraries in 1992, several experimental embodiments of the technology have been reduced to practice. This review provides a historical account of important milestones in the development of DNA-encoded chemical libraries, a survey of relevant ongoing research activities, and a glimpse into the future.
Keywords: DNA-encoded chemical libraries; combinatorial chemistry; drug discovery.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

