Proteoglycans as Immunomodulators of the Innate Immune Response to Lung Infection
- PMID: 29328866
- PMCID: PMC5958380
- DOI: 10.1369/0022155417751880
Proteoglycans as Immunomodulators of the Innate Immune Response to Lung Infection
Abstract
Proteoglycans (PGs) are complex, multifaceted molecules that participate in diverse interactions vital for physiological and pathological processes. As structural components, they provide a scaffold for cells and structural organization that helps define tissue architecture. Through interactions with water, PGs enable molecular and cellular movement through tissues. Through selective ionic interactions with growth factors, chemokines, cytokines, and proteases, PGs facilitate the ability of these soluble ligands to regulate intracellular signaling events and to influence the inflammatory response. In addition, recent findings now demonstrate that PGs can activate danger-associated molecular patterns (DAMPs) and other signaling pathways to influence production of many of these soluble ligands, indicating a more direct role for PGs in influencing the immune response and tissue inflammation. This review will focus on PGs that are selectively expressed during lung inflammation and will examine the novel emerging concept of PGs as immunomodulatory regulators of the innate immune responses in lungs.
Keywords: extracellular matrix; innate immunity; pulmonary inflammation; versican.
Conflict of interest statement
Figures

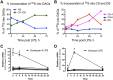




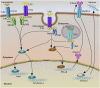
References
-
- Lin X, Wei G, Shi Z, Dryer L, Esko JD, Wells DE, Matzuk MM. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311. - PubMed
-
- Ringvall M, Ledin J, Holmborn K, van Kuppevelt T, Ellin F, Eriksson I, Olofsson AM, Kjellén L, Forsberg E. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2000;275:25926–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

