Development of a Cytocompatible Scaffold from Pig Immature Testicular Tissue Allowing Human Sertoli Cell Attachment, Proliferation and Functionality
- PMID: 29329231
- PMCID: PMC5796176
- DOI: 10.3390/ijms19010227
Development of a Cytocompatible Scaffold from Pig Immature Testicular Tissue Allowing Human Sertoli Cell Attachment, Proliferation and Functionality
Abstract
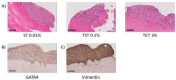
Cryopreservation of immature testicular tissue before chemo/radiotherapy is the only option to preserve fertility of cancer-affected prepubertal boys. To avoid reintroduction of malignant cells, development of a transplantable scaffold by decellularization of pig immature testicular tissue (ITT) able to support decontaminated testicular cells could be an option for fertility restoration in these patients. We, therefore, compared decellularization protocols to produce a cytocompatible scaffold. Fragments of ITT from 15 piglets were decellularized using three protocols: sodium dodecyl sulfate (SDS)-Triton (ST), Triton-SDS-Triton (TST) and trypsin 0.05%/ethylenediaminetetraacetic acid (EDTA) 0.02%-Triton (TET) with varying detergent concentrations. All protocols were able to lower DNA levels. Collagen retention was demonstrated in all groups except ST 1%, and a significant decrease in glycosaminoglycans was observed in the TST 1% and TET 1% groups. When Sertoli cells (SCs) were cultured with decellularized tissue, no signs of cytotoxicity were detected. A higher SC proliferation rate and greater stem cell factor secretion were observed than with SCs cultured without scaffold. ST 0.01% and TET 3% conditions offered the best compromise in terms of DNA elimination and extracellular matrix (ECM) preservation, while ensuring good attachment, proliferation and functionality of human SCs. This study demonstrates the potential of using decellularized pig ITT for human testicular tissue engineering purposes.
Keywords: decellularization; decellularized tissue; extracellular matrix; fertility preservation; immature testicular tissue; regenerative medicine; scaffold; testicular organoid; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

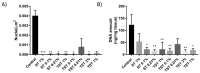


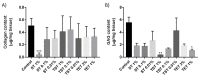

References
-
- Gatta G., Botta L., Rossi S., Aareleid T., Bielska-Lasota M., Clavel J., Dimitrova N., Jakab Z., Kaatsch P., Lacour B., et al. Childhood cancer survival in Europe 1999–2007: Results of eurocare-5—A population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

