Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review
- PMID: 29329257
- PMCID: PMC6017056
- DOI: 10.3390/molecules23010134
Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review
Abstract
Pyrazole and its derivatives are considered a pharmacologically important active scaffold that possesses almost all types of pharmacological activities. The presence of this nucleus in pharmacological agents of diverse therapeutic categories such as celecoxib, a potent anti-inflammatory, the antipsychotic CDPPB, the anti-obesity drug rimonabant, difenamizole, an analgesic, betazole, a H2-receptor agonist and the antidepressant agent fezolamide have proved the pharmacological potential of the pyrazole moiety. Owing to this diversity in the biological field, this nucleus has attracted the attention of many researchers to study its skeleton chemically and biologically. This review highlights the different synthesis methods and the pharmacological properties of pyrazole derivatives. Studies on the synthesis and biological activity of pyrazole derivatives developed by many scientists around the globe are reported.
Keywords: biological activities; pyrazole derivatives; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









































































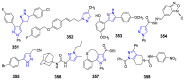






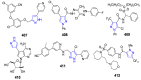




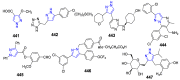





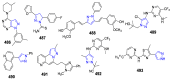



References
-
- Ansari A., Ali A., Asif M. biologically active pyrazole derivatives. New J. Chem. 2017;41:16–41. doi: 10.1039/C6NJ03181A. - DOI
-
- Steinbach G., Lynch P.M., Robin K.S.P., Wallace M.H., Hawk E., Gordon G.B., Wakabayashi N., Saunders B., Shen Y., Fujimura T., Su L.-K., Levin A.B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. - DOI - PubMed
-
- Uslaner J.M., Parmentier-Batteur S., Flick R.B., Surles N.O., Lam J.S., McNaughton C.H. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition memory is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

