Efficacy and safety of combined treatment of miniscalpel acupuncture and non-steroidal anti-inflammatory drugs: an assessor-blinded randomized controlled pilot study
- PMID: 29329551
- PMCID: PMC5766990
- DOI: 10.1186/s13063-017-2418-1
Efficacy and safety of combined treatment of miniscalpel acupuncture and non-steroidal anti-inflammatory drugs: an assessor-blinded randomized controlled pilot study
Abstract
Background: Chronic neck pain is a common musculoskeletal disease during the lifespan of an individual. With an increase in dependence on computer technology, the prevalence of chronic neck pain is expected to rise and this can lead to socioeconomic problems. We have designed the current pilot study to evaluate the efficacy and safety of miniscalpel acupuncture treatment combined with non-steroidal anti-inflammatory drugs (NSAIDs) in patients with chronic neck pain.
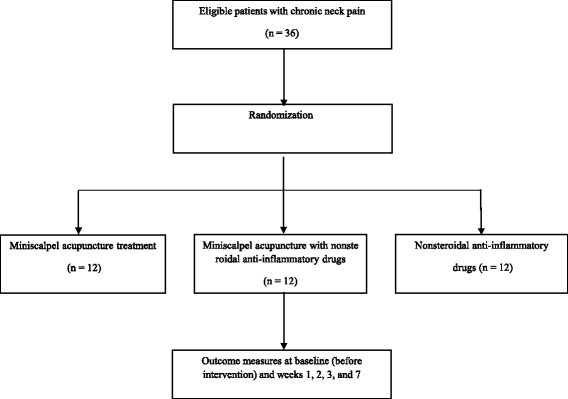
Methods: This seven-week clinical trial has been designed as an assessor-blinded, randomized controlled trial with three parallel arms. Thirty-six patients will be recruited and randomly allocated to three treatment groups: miniscalpel acupuncture treatment; NSAIDs; and miniscalpel acupuncture treatment combined with NSAIDs. Patients in the miniscalpel acupuncture and combined treatment groups will receive three sessions of miniscalpel acupuncture over a three-week period. Patients in the NSAIDs and combined treatment groups will receive zaltoprofen (one oral tablet, three times a day for three weeks). Primary and secondary outcomes will be measured at weeks 0 (baseline), 1, 2, 3 (primary end point), and 7 (four weeks after treatment completion) using the visual analogue scale and the Neck Disability Index, EuroQol 5-dimension questionnaire, and Patients' Global Impression of Change scale, respectively. Adverse events will also be recorded.
Discussion: This pilot study will provide a basic foundation for a future large-scale trial as well as information about the feasibility of miniscalpel acupuncture treatment combined with NSAIDs for chronic neck pain.
Trial registration: Korean Clinical Research Information Service registry, KCT0002258 . Registered on 9 March 2017.
Keywords: Chronic neck pain; Miniscalpel acupuncture; Non-steroidal anti-inflammatory drugs; Pilot study.
Conflict of interest statement
Ethics approval and consent to participate
The trial has been registered with the Korean Clinical Research Information Service registry (KCT0002258) and has been approved by the Institutional Review Board (IRB) of Daegu Oriental Hospital (DHUMC-D-17001-ANS-01). Any modifications in the study will be reported to the IRB. Written informed consent will be obtained from each participant before the initiation of any treatment.
Consent for publication
The study findings will be published in open-access journals and presented at national and international conferences.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

