PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway
- PMID: 29329563
- PMCID: PMC5767014
- DOI: 10.1186/s12989-018-0240-x
PM2.5-induced oxidative stress increases intercellular adhesion molecule-1 expression in lung epithelial cells through the IL-6/AKT/STAT3/NF-κB-dependent pathway
Abstract
Background: Epidemiological studies have shown that ambient air pollution is closely associated with increased respiratory inflammation and decreased lung function. Particulate matters (PMs) are major components of air pollution that damages lung cells. However, the mechanisms remain to be elucidated. This study examines the effects of PMs on intercellular adhesion molecule-1 (ICAM-1) expression and the related mechanisms in vitro and in vivo.
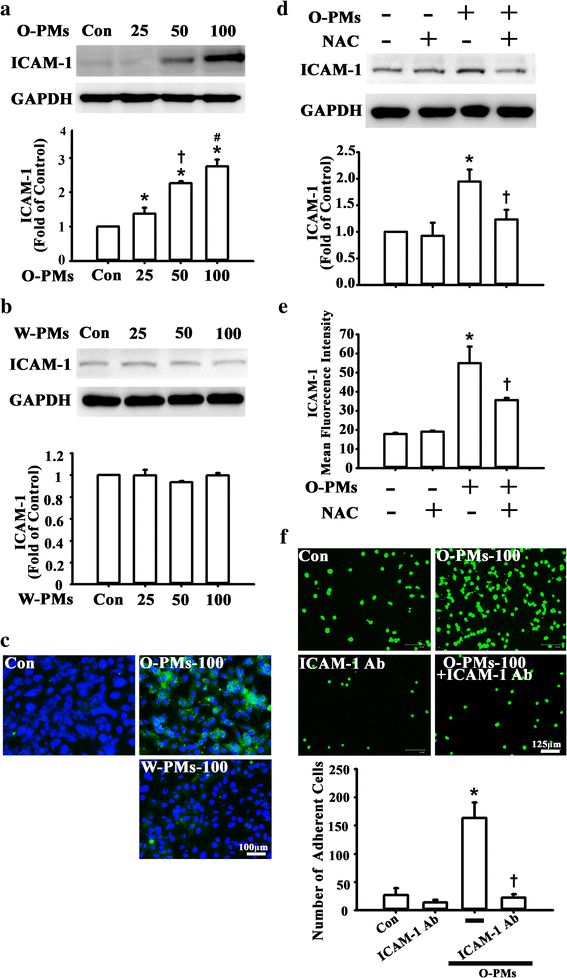
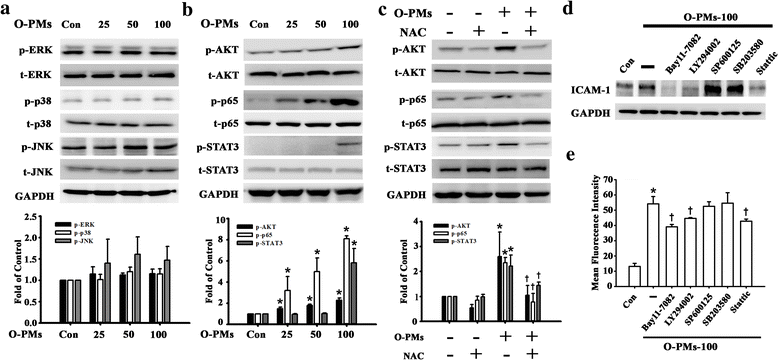
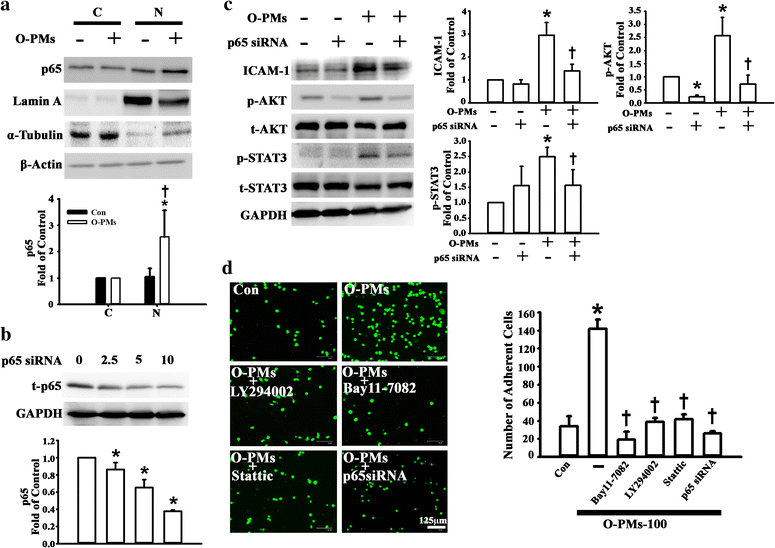
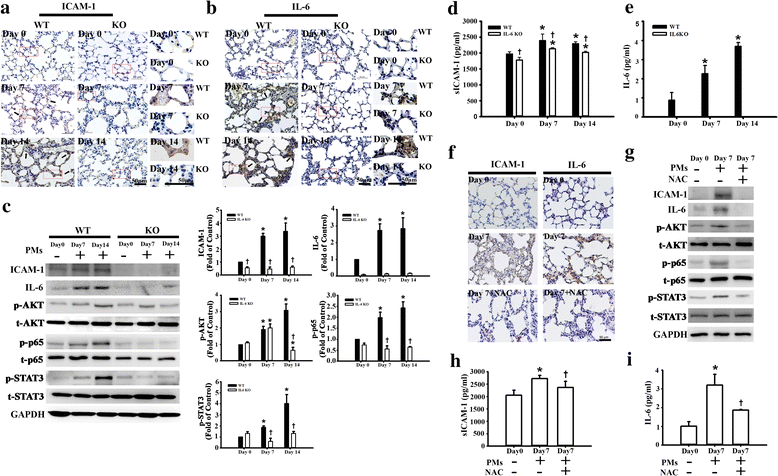
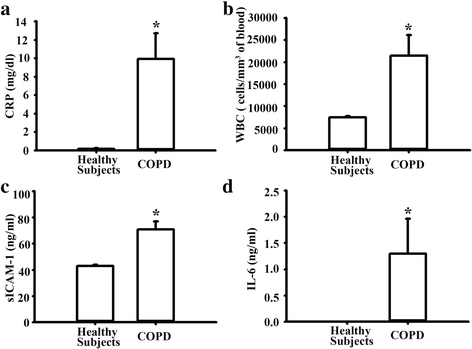
Result: The cytotoxicity, reactive oxygen species (ROS) generation, and monocyte adherence to A549 cells were more severely affected by treatment with O-PMs (organic solvent-extractable fraction of SRM1649b) than with W-PMs (water-soluble fraction of SRM1649b). We observed a significant increase in ICAM-1 expression by O-PMs, but not W-PMs. O-PMs also induced the phosphorylation of AKT, p65, and STAT3. Pretreating A549 cells with N-acetyl cysteine (NAC), an antioxidant, attenuated O-PMs-induced ROS generation, the phosphorylation of the mentioned kinases, and the expression of ICAM-1. Furthermore, an AKT inhibitor (LY294002), NF-κB inhibitor (BAY11-7082), and STAT3 inhibitor (Stattic) significantly down-regulated O-PMs-induced ICAM-1 expression as well as the adhesion of U937 cells to epithelial cells. Interleukin-6 (IL-6) was the most significantly changed cytokine in O-PMs-treated A549 cells according to the analysis of the cytokine antibody array. The IL-6 receptor inhibitor tocilizumab (TCZ) and small interfering RNA for IL-6 significantly reduced ICAM-1 secretion and expression as well as the reduction of the AKT, p65, and STAT3 phosphorylation in O-PMs-treated A549 cells. In addition, the intratracheal instillation of PMs significantly increased the levels of the ICAM-1 and IL-6 in lung tissues and plasma in WT mice, but not in IL-6 knockout mice. Pre-administration of NAC attenuated those PMs-induced adverse effects in WT mice. Furthermore, patients with chronic obstructive pulmonary disease (COPD) had higher plasma levels of ICAM-1 and IL-6 compared to healthy subjects.
Conclusion: These results suggest that PMs increase ICAM-1 expression in pulmonary epithelial cells in vitro and in vivo through the IL-6/AKT/STAT3/NF-κB signaling pathway.
Keywords: Inflammation; Intercellular adhesion molecule-1 (ICAM-1); Interleukin-6 (IL-6); Particulate matters (PMs); Reactive oxygen species (ROS).
Conflict of interest statement
Ethics approval
All animal procedures described in this study were conducted in accordance with the guidelines for the care and use of laboratory animals approved by National Taiwan University (ICCUC: 20,160,235). All participants provided informed written consent prior to participating in the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Taoyuan General Hospital (TYGH99025).
Consent for publication
No personal information is included in this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
-
- Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. doi: 10.1016/S0140-6736(13)62158-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

