Modified Pilates as an adjunct to standard physiotherapy care for urinary incontinence: a mixed methods pilot for a randomised controlled trial
- PMID: 29329567
- PMCID: PMC5767028
- DOI: 10.1186/s12905-017-0503-y
Modified Pilates as an adjunct to standard physiotherapy care for urinary incontinence: a mixed methods pilot for a randomised controlled trial
Abstract
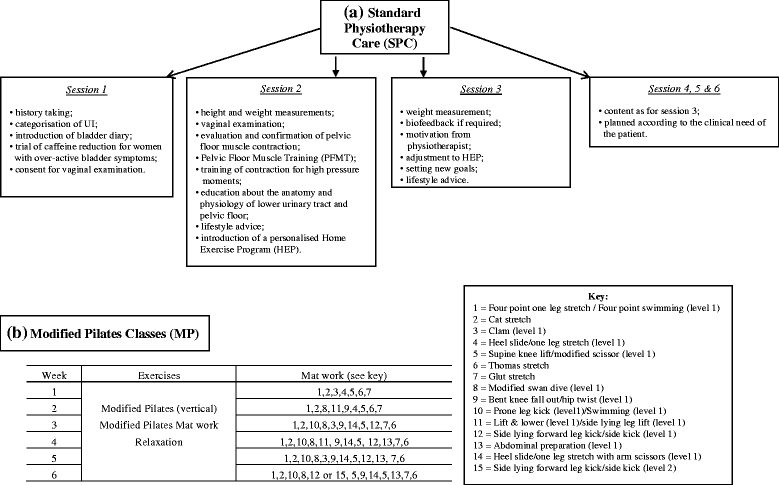
Background: Urinary incontinence (UI) is a distressing condition affecting at least 5 million women in England and Wales. Traditionally, physiotherapy for UI comprises pelvic floor muscle training, but although evidence suggests this can be effective it is also recognised that benefits are often compromised by patient motivation and commitment. In addition, there is increasing recognition that physical symptoms alone are poor indicators of the impact of incontinence on individuals' lives. Consequently, more holistic approaches to the treatment of UI, such as Modified Pilates (MP) have been recommended. This study aimed to provide preliminary findings about the effectiveness of a 6-week course of MP classes as an adjunct to standard physiotherapy care for UI, and to test the feasibility of a randomised controlled trial (RCT) design.
Methods: The study design was a single centre pilot RCT, plus qualitative interviews. 73 women referred to Women's Health Physiotherapy Services for UI at Colchester Hospital University NHS Foundation Trust were randomly assigned to two groups: a 6-week course of MP classes in addition to standard physiotherapy care (intervention) or standard physiotherapy care only (control). Main outcome measures were self-reported UI, quality of life and self-esteem at baseline (T1), completion of treatment (T2), and 5 months after randomisation (T3). Qualitative interviews were conducted with a subgroup at T2 and T3. Due to the nature of the intervention blinding of participants, physiotherapists and researchers was not feasible.
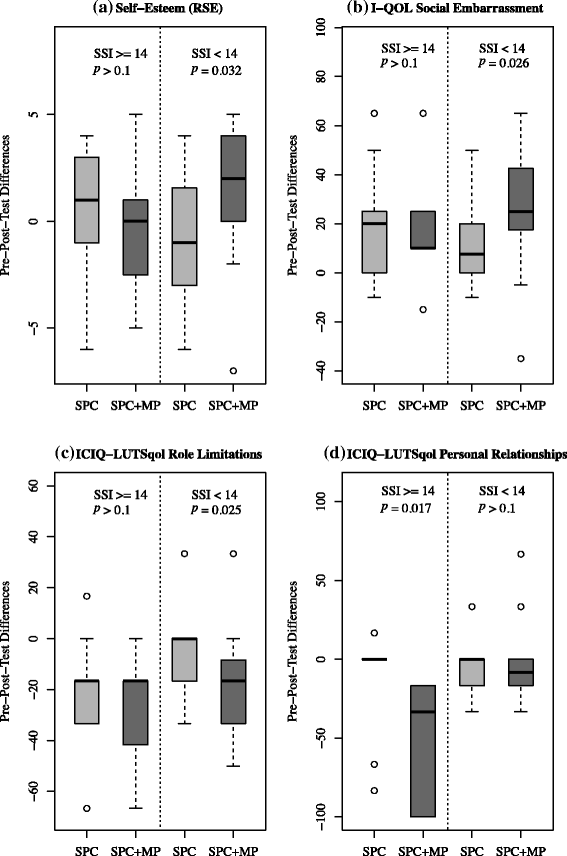
Results: Post-intervention data revealed a range of benefits for women who attended MP classes and who had lower symptom severity at baseline: improved self-esteem (p = 0.032), decreased social embarrassment (p = 0.026) and lower impact on normal daily activities (p = 0.025). In contrast, women with higher symptom severity showed improvement in their personal relationships (p = 0.017). Qualitative analysis supported these findings and also indicated that MP classes could positively influence attitudes to exercise, diet and wellbeing.
Conclusions: A definitive RCT is feasible but will require a large sample size to inform clinical practice.
Trial registration: ISRCTN74075972 Registered 12/12/12 (Retrospectively registered).
Keywords: Mixed methods; Modified Pilates; Pelvic floor muscle training; Physiotherapy; Randomised controlled trial; Urinary incontinence.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the NRES East of England Hertfordshire Ethics Committee Reference 12/EE/0241 on 18 July 2012, with a substantial amendment approved 24 June 2013. Written consent, including the voluntary nature of participation and the right to withdraw without medical care being affected, was obtained from each participant by the Research Physiotherapist.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- National Institute for Health and Care Excellence. The management of urinary incontinence in women. NICE clinical guidelines 40. Holborn London. 2013. https://www.nice.org.uk/guidance/cg171.
-
- Sinclair AJ, Ramsay IN. Review: the psychosocial impact of urinary incontinence in women. The Obstetrician & Gynaecologist. 2011;13:143–8. http://dx.doi.org/10.1576/toag.13.3.143.27665. - DOI
-
- National Institute for Health and Care Excellence. New NICE guidelines launched to improve treatment & care for millions of women suffering in silence. Holborn London. Ref: 2006/049; 2006. https://www.nice.org.uk/guidance/CG40.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

