Mesenchymal stem cell-macrophage crosstalk and bone healing
- PMID: 29329642
- PMCID: PMC6028312
- DOI: 10.1016/j.biomaterials.2017.12.025
Mesenchymal stem cell-macrophage crosstalk and bone healing
Abstract
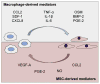
Recent research has brought about a clear understanding that successful fracture healing is based on carefully coordinated cross-talk between inflammatory and bone forming cells. In particular, the key role that macrophages play in the recruitment and regulation of the differentiation of mesenchymal stem cells (MSCs) during bone regeneration has been brought to focus. Indeed, animal studies have comprehensively demonstrated that fractures do not heal without the direct involvement of macrophages. Yet the exact mechanisms by which macrophages contribute to bone regeneration remain to be elucidated. Macrophage-derived paracrine signaling molecules such as Oncostatin M, Prostaglandin E2 (PGE2), and Bone Morphogenetic Protein-2 (BMP2) have been shown to play critical roles; however the relative importance of inflammatory (M1) and tissue regenerative (M2) macrophages in guiding MSC differentiation along the osteogenic pathway remains poorly understood. In this review, we summarize the current understanding of the interaction of macrophages and MSCs during bone regeneration, with the emphasis on the role of macrophages in regulating bone formation. The potential implications of aging to this cellular cross-talk are reviewed. Emerging treatment options to improve facture healing by utilizing or targeting MSC-macrophage crosstalk are also discussed.
Keywords: Bone Morphogenetic Protein-2; Fracture healing; Macrophage; Mesenchymal stem cell; Oncostatin M; Prostaglandin E2.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures



References
-
- C. Control. National Hospital Ambulatory Medical Care Survey: 2013 Emergency Department Summary Tables. 2013 Available from: https://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2013_ed_web_tables.pdf.
-
- Miranda M, Moon M. Treatment strategy for nonunions and malunions. In: Stannard J, Schmidt P, editors. Surgical Treatment of Orthopaedic Trauma. Thieme; New York: 2007. pp. 77–100.
-
- Hak DJ, Fitzpatrick D, Bishop JA, Marsh JL, Tilp S, Schnettler R, Simpson H, Alt V. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014 Jun;45(Suppl 2):S3–S7. - PubMed
-
- Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002 Sep;20(5):1091–1098. - PubMed
-
- Uhthoff HK, Rahn BA. Healing patterns of metaphyseal fractures. Clin Orthop Relat Res. 1981 Oct;(160):295–303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

