16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing
- PMID: 29329870
- PMCID: PMC5774150
- DOI: 10.1016/j.foodchem.2017.12.034
16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing
Abstract
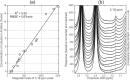
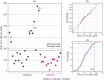
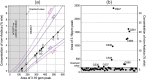
High-field and low-field proton NMR spectroscopy were used to analyse lipophilic extracts from ground roast coffees. Using a sample preparation method that produced concentrated extracts, a small marker peak at 3.16 ppm was observed in 30 Arabica coffees of assured origin. This signal has previously been believed absent from Arabicas, and has been used as a marker for detecting adulteration with robusta. Via 2D 600 MHz NMR and LC-MS, 16-O-methylcafestol and 16-O-methylkahweol were detected for the first time in Arabica roast coffee and shown to be responsible for the marker peak. Using low-field NMR, robusta in Arabica could be detected at levels of the order of 1-2% w/w. A surveillance study of retail purchased "100% Arabica" coffees found that 6 out of 60 samples displayed the 3.16 ppm marker signal to a degree commensurate with adulteration at levels of 3-30% w/w.
Keywords: Adulteration; Arabica; Authentication; Coffee; Low-field; NMR; Robusta; Species; Spectroscopy.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Aga E., Bekele E., Bryngelsson T. Inter-simple sequence repeat (ISSR) variation in forest coffee trees (Coffea arabica L.) populations from Ethiopia. Genetica. 2005;124(2–3):213–221. - PubMed
-
- Aga E., Bryngelsson T., Bekele E., Salomon B. Genetic diversity of forest arabica coffee (Coffea arabica L.) in Ethiopia as revealed by random amplified polymorphic DNA (RAPD) analysis. Hereditas. 2003;138(1):36–46. - PubMed
-
- Anthony F., Bertrand B., Quiros O., Wilches A., Lashermes P., Berthaud J. Genetic diversity of wild coffee (Coffea arabica L.) using molecular markers. Euphytica. 2001;118(1):53–65.
-
- Belitz H.D., Grosch W., Schieberle P. 4 ed. Spinger; Berlin Heidelberg: 2009. Food chemistry.
-
- Bonnlander B., Wunnecke H., Winterhalter P. Rapid and sensitive detection of robusta traces in blends of raw and roasted coffee. Lebensmittelchemie. 2007;61:79–80.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

