Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study
- PMID: 29329957
- PMCID: PMC5871563
- DOI: 10.1016/S1473-3099(18)30002-1
Safety and tolerability of a novel, polyclonal human anti-MERS coronavirus antibody produced from transchromosomic cattle: a phase 1 randomised, double-blind, single-dose-escalation study
Abstract
Background: Middle East respiratory syndrome (MERS) is a severe respiratory illness with an overall mortality of 35%. There is no licensed or proven treatment. Passive immunotherapy approaches are being developed to prevent and treat several human medical conditions where alternative therapeutic options are absent. We report the safety of a fully human polyclonal IgG antibody (SAB-301) produced from the hyperimmune plasma of transchromosomic cattle immunised with a MERS coronavirus vaccine.
Methods: We did a phase 1 double-blind, placebo-controlled, single-dose escalation trial at the National Institutes of Health Clinical Center. We recruited healthy participants aged 18-60 years who had normal laboratory parameters at enrolment, a body-mass index of 19-32 kg/m2, and a creatinine clearance of 70 mL/min or more, and who did not have any chronic medical problems that required daily oral medications, a positive rheumatoid factor (≥15 IU/mL), IgA deficiency (<7 mg/dL), or history of allergy to intravenous immunoglobulin or human blood products. Participants were randomly assigned by a computer-generated table, made by a masked pharmacist, to one of six cohorts (containing between three and ten participants each). Cohorts 1 and 2 had three participants, randomly assigned 2:1 to receive active drug SAB-301 versus normal saline placebo; cohorts 3 and 4 had six participants randomised 2:1; and cohorts 5 and 6 had ten participants, randomised 4:1. Participants received 1 mg/kg, 2·5 mg/kg, 5 mg/kg, 10 mg/kg, 20 mg/kg, or 50 mg/kg of SAB-301, or equivalent volume placebo (saline control), on day 0, and were followed up by clinical, laboratory, and pharmacokinetic assessments on days 1, 3, 7, 21, 42, and 90. The primary outcome was safety, and immunogenicity was a secondary outcome. We analysed the intention-to-treat population. This trial is registered with ClinicalTrials.gov, number NCT02788188.
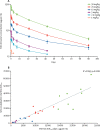
Findings: Between June 2, 2016, and Jan 4, 2017, we screened 43 participants, of whom 38 were eligible and randomly assigned to receive SAB-301 (n=28) or placebo (n=10). 97 adverse events were reported: 64 adverse events occurred in 23 (82%) of 28 participants receiving SAB-301 (mean 2·3 adverse events per participant). 33 adverse events occurred in all ten participants receiving placebo (mean 3·3 adverse events per participant). The most common adverse events were headache (n=6 [21%] in participants who received SAB-301 and n=2 [20%] in those receiving placebo), albuminuria (n=5 [18%] vs n=2 [20%]), myalgia (n=3 [11%] vs n=1 [10%]), increased creatine kinase (n=3 [11%] vs 1 [10%]), and common cold (n=3 [11%] vs n=2 [20%]). There was one serious adverse event (hospital admission for suicide attempt) in one participant who received 50 mg/kg of SAB-301. The area under the concentration-time curve (AUC) in the 50 mg/kg dose (27 498 μg × days per mL) is comparable to the AUC that was associated with efficacy in a preclinical model.
Interpretation: Single infusions of SAB-301 up to 50 mg/kg appear to be safe and well tolerated in healthy participants. Human immunoglobulin derived from transchromosomic cattle could offer a new platform technology to produce fully human polyclonal IgG antibodies for other medical conditions.
Funding: National Institute of Allergy and Infectious Diseases, National Institutes of Health, and Biomedical Advanced Research and Development Authority.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Herd immunity: hyperimmune globulins for the 21st century.Lancet Infect Dis. 2018 Apr;18(4):361-363. doi: 10.1016/S1473-3099(18)30003-3. Epub 2018 Jan 9. Lancet Infect Dis. 2018. PMID: 29329956 Free PMC article. No abstract available.
References
-
- WHO Middle East respiratory syndrome coronavirus (MERS-CoV) Sept 27, 2017. http://www.who.int/emergencies/mers-cov/en/ (accessed Nov 27, 2017).
-
- Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22:521–526. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

