Effect of Plasmid Design and Type of Integration Event on Recombinant Protein Expression in Pichia pastoris
- PMID: 29330186
- PMCID: PMC5835746
- DOI: 10.1128/AEM.02712-17
Effect of Plasmid Design and Type of Integration Event on Recombinant Protein Expression in Pichia pastoris
Abstract
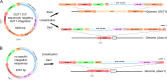
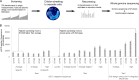
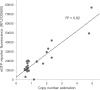
Pichia pastoris (syn. Komagataella phaffii) is one of the most common eukaryotic expression systems for heterologous protein production. Expression cassettes are typically integrated in the genome to obtain stable expression strains. In contrast to Saccharomyces cerevisiae, where short overhangs are sufficient to target highly specific integration, long overhangs are more efficient in P. pastoris and ectopic integration of foreign DNA can occur. Here, we aimed to elucidate the influence of ectopic integration by high-throughput screening of >700 transformants and whole-genome sequencing of 27 transformants. Different vector designs and linearization approaches were used to mimic the most common integration events targeted in P. pastoris Fluorescence of an enhanced green fluorescent protein (eGFP) reporter protein was highly uniform among transformants when the expression cassettes were correctly integrated in the targeted locus. Surprisingly, most nonspecifically integrated transformants showed highly uniform expression that was comparable to specific integration, suggesting that nonspecific integration does not necessarily influence expression. However, a few clones (<10%) harboring ectopically integrated cassettes showed a greater variation spanning a 25-fold range, surpassing specifically integrated reference strains up to 6-fold. High-expression strains showed a correlation between increased gene copy numbers and high reporter protein fluorescence levels. Our results suggest that for comparing expression levels between strains, the integration locus can be neglected as long as a sufficient numbers of transformed strains are compared. For expression optimization of highly expressible proteins, increasing copy number appears to be the dominant positive influence rather than the integration locus, genomic rearrangements, deletions, or single-nucleotide polymorphisms (SNPs).IMPORTANCE Yeasts are commonly used as biotechnological production hosts for proteins and metabolites. In the yeast Saccharomyces cerevisiae, expression cassettes carrying foreign genes integrate highly specifically at the targeted sites in the genome. In contrast, cassettes often integrate at random genomic positions in nonconventional yeasts, such as Pichia pastoris (syn. Komagataella phaffii). Hence, cells from the same transformation event often behave differently, with significant clonal variation necessitating the screening of large numbers of strains. The importance of this study is that we systematically investigated the influence of integration events in more than 700 strains. Our findings provide novel insight into clonal variation in P. pastoris and, thus, how to avoid pitfalls and obtain reliable results. The underlying mechanisms may also play a role in other yeasts and hence could be generally relevant for recombinant yeast protein production strains.
Keywords: Pichia pastoris; genome analysis; integration; protein expression.
Copyright © 2018 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

