Is placebo response in antidepressant trials rising or not? A reanalysis of datasets to conclude this long-lasting controversy
- PMID: 29330216
- PMCID: PMC10270408
- DOI: 10.1136/eb-2017-102827
Is placebo response in antidepressant trials rising or not? A reanalysis of datasets to conclude this long-lasting controversy
Abstract
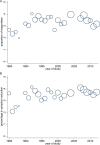
It had long been believed that placebo response rates in antidepressant trials have been increasing and that they were responsible for rising numbers of so-called failed antidepressant trials. Two recent systematic reviews examined this issue and reached completely opposite findings. Furukawa and colleagues in a paper published in 2016 found that the placebo response rates are stable since 1991 and the apparent increase up to 2000 was confounded by changes in trial design features. By contrast, Khan and colleagues more recently concluded that placebo response rates had grown steadily in the past 30 years. The two reviews differed in the datasets they used, definitions of placebo response and statistical analyses. In this perspective article, we examined if such differences were responsible for the two reviews' contrasting conclusions. Our reanalyses confirmed our previous results. We found that in any dataset and for any placebo response definition, there was no increase in placebo response over the years when the analysis was adjusted for the confounders related to study design features or when it was limited to studies published after 1990s. We conclude that placebo response in antidepressant trials has remained stable for the past 25 years, during which time the large majority of the studies have come to share similar design features.
Keywords: depression & mood disorders.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: TAF has received lecture fees from Eli Lilly, Janssen, Meiji, MSD, Pfizer and Tanabe-Mitsubishi and consultancy fees from Takeda Science Foundation. He has received royalties from Igaku-Shoin and Nihon Bunka Kagaku-sha publishers. He has received grant or research support from Mochida and Tanabe-Mitsubishi. AC was expert witness for Accord Healthcare for a patent issue about quetiapine extended release. SL has received honoraria for lectures from Eli Lilly, Lundbeck (Institute), Pfizer, Janssen, BMS, Johnson and Johnson, Otsuka, Roche, SanofiAventis, ICON, Abbvie, AOP Orphan, Servier; for consulting/advisory boards from Roche, Janssen, Lundbeck, Eli Lilly, Otsuka, TEVA; for the preparation of educational material and publications from Lundbeck Institute and Roche. Eli Lilly has provided medication for a clinical trial led by SL as principal investigator. All the other authors declare no competing interests.
Figures
Comment on
-
Placebo response rates in antidepressant trials: a systematic review of published and unpublished double-blind randomised controlled studies.Lancet Psychiatry. 2016 Nov;3(11):1059-1066. doi: 10.1016/S2215-0366(16)30307-8. Epub 2016 Oct 7. Lancet Psychiatry. 2016. PMID: 27726982
-
Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013.World Psychiatry. 2017 Jun;16(2):181-192. doi: 10.1002/wps.20421. World Psychiatry. 2017. PMID: 28498591 Free PMC article.
References
-
- Walsh BT, Seidman SN, Sysko R, et al. . Placebo response in studies of major depression: variable, substantial, and growing. JAMA 2002;287:1840–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous