Centriole Overduplication is the Predominant Mechanism Leading to Centrosome Amplification in Melanoma
- PMID: 29330283
- PMCID: PMC5835182
- DOI: 10.1158/1541-7786.MCR-17-0197
Centriole Overduplication is the Predominant Mechanism Leading to Centrosome Amplification in Melanoma
Abstract
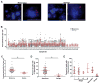
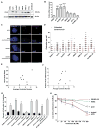
Centrosome amplification (CA) is common in cancer and can arise by centriole overduplication or by cell doubling events, including the failure of cell division and cell-cell fusion. To assess the relative contributions of these two mechanisms, the number of centrosomes with mature/mother centrioles was examined by immunofluorescence in a tissue microarray of human melanomas and benign nevi (n = 79 and 17, respectively). The centrosomal protein 170 (CEP170) was used to identify centrosomes with mature centrioles; this is expected to be present in most centrosomes with cell doubling, but on fewer centrosomes with overduplication. Using this method, it was determined that the majority of CA in melanoma can be attributed to centriole overduplication rather than cell doubling events. As Polo-like kinase 4 (PLK4) is the master regulator of centriole duplication, the hypothesis that PLK4 overexpression contributes to centriole overduplication was evaluated. PLK4 is significantly overexpressed in melanoma compared with benign nevi and in a panel of human melanoma cell lines (A375, Hs294T, G361, WM35, WM115, 451Lu, and SK-MEL-28) compared with normal human melanocytes. Interestingly, although PLK4 expression did not correlate with CA in most cases, treatment of melanoma cells with a selective small-molecule PLK4 inhibitor (centrinone B) significantly decreased cell proliferation. The antiproliferative effects of centrinone B were also accompanied by induction of apoptosis.Implications: This study demonstrates that centriole overduplication is the predominant mechanism leading to centrosome amplification in melanoma and that PLK4 should be further evaluated as a potential therapeutic target for melanoma treatment. Mol Cancer Res; 16(3); 517-27. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

