Cutting Edge: Plasmodium falciparum Induces Trained Innate Immunity
- PMID: 29330325
- PMCID: PMC5927587
- DOI: 10.4049/jimmunol.1701010
Cutting Edge: Plasmodium falciparum Induces Trained Innate Immunity
Abstract
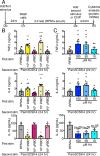
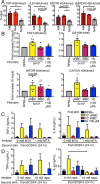
Malarial infection in naive individuals induces a robust innate immune response. In the recently described model of innate immune memory, an initial stimulus primes the innate immune system to either hyperrespond (termed training) or hyporespond (tolerance) to subsequent immune challenge. Previous work in both mice and humans demonstrated that infection with malaria can both serve as a priming stimulus and promote tolerance to subsequent infection. In this study, we demonstrate that initial stimulation with Plasmodium falciparum-infected RBCs or the malaria crystal hemozoin induced human adherent PBMCs to hyperrespond to subsequent ligation of TLR2. This hyperresponsiveness correlated with increased H3K4me3 at important immunometabolic promoters, and these epigenetic modifications were also seen in Kenyan children naturally infected with malaria. However, the use of epigenetic and metabolic inhibitors indicated that the induction of trained immunity by malaria and its ligands may occur via a previously unrecognized mechanism(s).
Copyright © 2018 by The American Association of Immunologists, Inc.
Figures

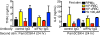
References
-
- Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol. 2014;14:744–757. - PubMed
-
- Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, Gazzinelli RT, Golenbock DT. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci USA. 2007;104:1919–1924. - PMC - PubMed
-
- Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, Barber GN, Gazzinelli RT, Fitzgerald KA, Golenbock DT. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35:194–207. - PMC - PubMed
-
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends in Immunology. 2003;24:491–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

