The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes
- PMID: 29330340
- PMCID: PMC5875945
- DOI: 10.1681/ASN.2017060627
The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes
Abstract
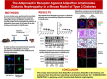
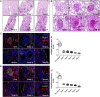
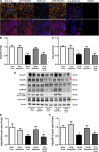
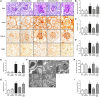
Adiponectin exerts renoprotective effects against diabetic nephropathy (DN) by activating the AMP-activated protein kinase (AMPK)/peroxisome proliferative-activated receptor-α (PPARα) pathway through adiponectin receptors (AdipoRs). AdipoRon is an orally active synthetic adiponectin receptor agonist. We investigated the expression of AdipoRs and the associated intracellular pathways in 27 patients with type 2 diabetes and examined the effects of AdipoRon on DN development in male C57BLKS/J db/db mice, glomerular endothelial cells (GECs), and podocytes. The extent of glomerulosclerosis and tubulointerstitial fibrosis correlated with renal function deterioration in human kidneys. Expression of AdipoR1, AdipoR2, and Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKKβ) and numbers of phosphorylated liver kinase B1 (LKB1)- and AMPK-positive cells significantly decreased in the glomeruli of early stage human DN. AdipoRon treatment restored diabetes-induced renal alterations in db/db mice. AdipoRon exerted renoprotective effects by directly activating intrarenal AdipoR1 and AdipoR2, which increased CaMKKβ, phosphorylated Ser431LKB1, phosphorylated Thr172AMPK, and PPARα expression independently of the systemic effects of adiponectin. AdipoRon-induced improvement in diabetes-induced oxidative stress and inhibition of apoptosis in the kidneys ameliorated relevant intracellular pathways associated with lipid accumulation and endothelial dysfunction. In high-glucose-treated human GECs and murine podocytes, AdipoRon increased intracellular Ca2+ levels that activated a CaMKKβ/phosphorylated Ser431LKB1/phosphorylated Thr172AMPK/PPARα pathway and downstream signaling, thus decreasing high-glucose-induced oxidative stress and apoptosis and improving endothelial dysfunction. AdipoRon further produced cardioprotective effects through the same pathway demonstrated in the kidney. Our results show that AdipoRon ameliorates GEC and podocyte injury by activating the intracellular Ca2+/LKB1-AMPK/PPARα pathway, suggesting its efficacy for treating type 2 diabetes-associated DN.
Keywords: AdipoRon; Lipotoxicity; diabetic nephropathy; oxidative stress.
Copyright © 2018 by the American Society of Nephrology.
Figures









References
-
- Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M: Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem 280: 32317–32325, 2005 - PubMed
-
- Yilmaz M, Hotamisligil GS: Damned if you do, damned if you don’t: The conundrum of adipose tissue vascularization. Cell Metab 17: 7–9, 2013 - PubMed
-
- Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y: Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

