Pathogenesis of bone disease in multiple myeloma: from bench to bedside
- PMID: 29330358
- PMCID: PMC5802524
- DOI: 10.1038/s41408-017-0037-4
Pathogenesis of bone disease in multiple myeloma: from bench to bedside
Abstract
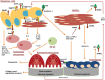
Osteolytic bone disease is the hallmark of multiple myeloma, which deteriorates the quality of life of myeloma patients, and it affects dramatically their morbidity and mortality. The basis of the pathogenesis of myeloma-related bone disease is the uncoupling of the bone-remodeling process. The interaction between myeloma cells and the bone microenvironment ultimately leads to the activation of osteoclasts and suppression of osteoblasts, resulting in bone loss. Several intracellular and intercellular signaling cascades, including RANK/RANKL/OPG, Notch, Wnt, and numerous chemokines and interleukins are implicated in this complex process. During the last years, osteocytes have emerged as key regulators of bone loss in myeloma through direct interactions with the myeloma cells. The myeloma-induced crosstalk among the molecular pathways establishes a positive feedback that sustains myeloma cell survival and continuous bone destruction, even when a plateau phase of the disease has been achieved. Targeted therapies, based on the better knowledge of the biology, constitute a promising approach in the management of myeloma-related bone disease and several novel agents are currently under investigation. Herein, we provide an insight into the underlying pathogenesis of bone disease and discuss possible directions for future studies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

