Phenotypic and Functional Characterization of Peripheral Sensory Neurons derived from Human Embryonic Stem Cells
- PMID: 29330377
- PMCID: PMC5766621
- DOI: 10.1038/s41598-017-19093-0
Phenotypic and Functional Characterization of Peripheral Sensory Neurons derived from Human Embryonic Stem Cells
Abstract
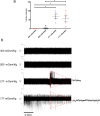
The dorsal root ganglia (DRG) consist of a multitude of sensory neuronal subtypes that function to relay sensory stimuli, including temperature, pressure, pain and position to the central nervous system. Our knowledge of DRG sensory neurons have been predominantly driven by animal studies and considerably less is known about the human DRG. Human embryonic stem cells (hESC) are valuable resource to help close this gap. Our previous studies reported an efficient system for deriving neural crest and DRG sensory neurons from hESC. Here we show that this differentiation system gives rise to heterogeneous populations of sensory neuronal subtypes as demonstrated by phenotypic and functional analyses. Furthermore, using microelectrode arrays the maturation rate of the hESC-derived sensory neuronal cultures was monitored over 8 weeks in culture, showing their spontaneous firing activities starting at about 12 days post-differentiation and reaching maximum firing at about 6 weeks. These studies are highly valuable for developing an in vitro platform to study the diversity of sensory neuronal subtypes found within the human DRG.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

