Human Placental-Derived Adherent Stromal Cells Co-Induced with TNF-α and IFN-γ Inhibit Triple-Negative Breast Cancer in Nude Mouse Xenograft Models
- PMID: 29330447
- PMCID: PMC5766494
- DOI: 10.1038/s41598-017-18428-1
Human Placental-Derived Adherent Stromal Cells Co-Induced with TNF-α and IFN-γ Inhibit Triple-Negative Breast Cancer in Nude Mouse Xenograft Models
Abstract
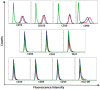
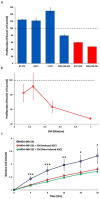
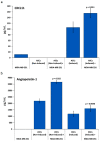
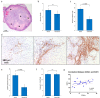
Culturing 3D-expanded human placental-derived adherent stromal cells (ASCs) in the presence of tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) transiently upregulated the secretion of numerous anti-proliferative, anti-angiogenic and pro-inflammatory cytokines. In a 3D-spheroid screening assay, conditioned medium from these induced-ASCs inhibited proliferation of cancer cell lines, including triple-negative breast cancer (TNBC) lines. In vitro co-culture studies of induced-ASCs with MDA-MB-231 human breast carcinoma cells, a model representing TNBC, supports a mechanism involving immunomodulation and angiogenesis inhibition. In vivo studies in nude mice showed that intramuscular administration of induced-ASCs halted MDA-MB-231 cell proliferation, and inhibited tumor progression and vascularization. Thirty percent of treated mice experienced complete tumor remission. Murine serum concentrations of the tumor-supporting cytokines Interleukin-6 (IL-6), Vascular endothelial growth factor (VEGF) and Granulocyte-colony stimulating factor (G-CSF) were lowered to naïve levels. A somatic mutation analysis identified numerous genes which could be screened in patients to increase a positive therapeutic outcome. Taken together, these results show that targeted changes in the secretion profile of ASCs may improve their therapeutic potential.
Conflict of interest statement
The authors are current and former employees and shareholders of Pluristem Ltd.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

