miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2
- PMID: 29330462
- PMCID: PMC5766605
- DOI: 10.1038/s41598-017-18739-3
miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2
Abstract
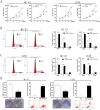
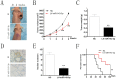
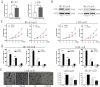
Osteosarcoma (OS) is the most common type of primary malignant bone tumor and mainly occurs in children and adolescent. Because of its early migration and invasion, OS has a poor prognosis. It has been reported that mircoRNAs (miRNAs) play a crucial role in the occurrence and development of multiple tumors. In this study, we identified the aberrant-expression of miR-143-3p in osteosarcoma and examined the role of miR-143-3p in OS development. Further, we searched the miR-143-3p target gene and verified its accuracy by luciferase experiments. Finally, we explored the relationship between miR-143-3p and FOS-Like antigen 2 (FOSL2). Our data indicated that miR-143-3p expression was substantially lower in OS tissues and cell-line compared with normal tissues, and was lower in patients with poor prognosis. In addition miR-143-3p inhibited OS cell proliferation and metastasis while promoting apoptosis. We next showed that FOSL2 was directly targeted by miR-143-3p and could reverse the inhibition caused by miR-143-3p. Finally, we found FOSL2 expression in OS cells was significantly higher compared with normal cells and negatively correlated with miR-143-3p. Thus, miR-143-3p directly and negatively targets FOSL2 to affect OS characteristics. This provides a new target for the treatment of OS and deserves further study.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
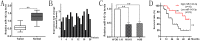


References
-
- Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am J Orthop (Belle Mead NJ) 2015;44:547. - PubMed
-
- Chang L, Shrestha S, LaChaud G, Scott MA, James AW. Review of microRNA in osteosarcoma and chondrosarcoma. Med Oncol. 2015;32:613. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

