Anti-correlated cortical networks arise from spontaneous neuronal dynamics at slow timescales
- PMID: 29330480
- PMCID: PMC5766587
- DOI: 10.1038/s41598-017-18097-0
Anti-correlated cortical networks arise from spontaneous neuronal dynamics at slow timescales
Abstract
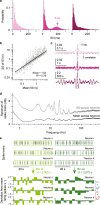
In the highly interconnected architectures of the cerebral cortex, recurrent intracortical loops disproportionately outnumber thalamo-cortical inputs. These networks are also capable of generating neuronal activity without feedforward sensory drive. It is unknown, however, what spatiotemporal patterns may be solely attributed to intrinsic connections of the local cortical network. Using high-density microelectrode arrays, here we show that in the isolated, primary somatosensory cortex of mice, neuronal firing fluctuates on timescales from milliseconds to tens of seconds. Slower firing fluctuations reveal two spatially distinct neuronal ensembles, which correspond to superficial and deeper layers. These ensembles are anti-correlated: when one fires more, the other fires less and vice versa. This interplay is clearest at timescales of several seconds and is therefore consistent with shifts between active sensing and anticipatory behavioral states in mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

