Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model
- PMID: 29331024
- PMCID: PMC6002225
- DOI: 10.1111/imm.12893
Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model
Abstract
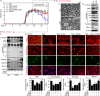
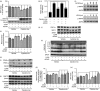
Recent studies report that loss and dysfunction of mitochondria and peroxisomes contribute to the myelin and axonal damage in multiple sclerosis (MS). In this study, we investigated the efficacy of a combination of lovastatin and AMP-activated protein kinase (AMPK) activator (AICAR) on the loss and dysfunction of mitochondria and peroxisomes and myelin and axonal damage in spinal cords, relative to the clinical disease symptoms, using a mouse model of experimental autoimmune encephalomyelitis (EAE, a model for MS). We observed that lovastatin and AICAR treatments individually provided partial protection of mitochondria/peroxisomes and myelin/axons, and therefore partial attenuation of clinical disease in EAE mice. However, treatment of EAE mice with the lovastatin and AICAR combination provided greater protection of mitochondria/peroxisomes and myelin/axons, and greater improvement in clinical disease compared with individual drug treatments. In spinal cords of EAE mice, lovastatin-mediated inhibition of RhoA and AICAR-mediated activation of AMPK cooperatively enhanced the expression of the transcription factors and regulators (e.g. PPARα/β, SIRT-1, NRF-1, and TFAM) required for biogenesis and the functions of mitochondria (e.g. OXPHOS, MnSOD) and peroxisomes (e.g. PMP70 and catalase). In summary, these studies document that oral medication with a combination of lovastatin and AICAR, which are individually known to have immunomodulatory effects, provides potent protection and repair of inflammation-induced loss and dysfunction of mitochondria and peroxisomes as well as myelin and axonal abnormalities in EAE. As statins are known to provide protection in progressive MS (Phase II study), these studies support that supplementation statin treatment with an AMPK activator may provide greater efficacy against MS.
Keywords: autoimmunity; experimental autoimmune encephalomyelitis/multiple sclerosis; neurodegeneration; neuroinflammation.
© 2018 John Wiley & Sons Ltd.
Figures



References
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359:1221–31. - PubMed
-
- Michel L, Larochelle C, Prat A. Update on treatments in multiple sclerosis. Presse Med 2015; 44:e137–51. - PubMed
-
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM et al A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med 2011; 17:495–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

