Terminal spreading depolarization and electrical silence in death of human cerebral cortex
- PMID: 29331091
- PMCID: PMC5901399
- DOI: 10.1002/ana.25147
Terminal spreading depolarization and electrical silence in death of human cerebral cortex
Abstract
Objective: Restoring the circulation is the primary goal in emergency treatment of cerebral ischemia. However, better understanding of how the brain responds to energy depletion could help predict the time available for resuscitation until irreversible damage and advance development of interventions that prolong this span. Experimentally, injury to central neurons begins only with anoxic depolarization. This potentially reversible, spreading wave typically starts 2 to 5 minutes after the onset of severe ischemia, marking the onset of a toxic intraneuronal change that eventually results in irreversible injury.
Methods: To investigate this in the human brain, we performed recordings with either subdural electrode strips (n = 4) or intraparenchymal electrode arrays (n = 5) in patients with devastating brain injury that resulted in activation of a Do Not Resuscitate-Comfort Care order followed by terminal extubation.
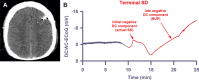
Results: Withdrawal of life-sustaining therapies produced a decline in brain tissue partial pressure of oxygen (pti O2 ) and circulatory arrest. Silencing of spontaneous electrical activity developed simultaneously across regional electrode arrays in 8 patients. This silencing, termed "nonspreading depression," developed during the steep falling phase of pti O2 (intraparenchymal sensor, n = 6) at 11 (interquartile range [IQR] = 7-14) mmHg. Terminal spreading depolarizations started to propagate between electrodes 3.9 (IQR = 2.6-6.3) minutes after onset of the final drop in perfusion and 13 to 266 seconds after nonspreading depression. In 1 patient, terminal spreading depolarization induced the initial electrocerebral silence in a spreading depression pattern; circulatory arrest developed thereafter.
Interpretation: These results provide fundamental insight into the neurobiology of dying and have important implications for survivable cerebral ischemic insults. Ann Neurol 2018;83:295-310.
© 2018 Authors Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures





References
-
- García JL, Anderson ML. Circulatory disorders and their effect on the brain In: Davis RL, Robertson DM, eds. Textbook of neuropathology. Baltimore, MD: Williams & Wilkins, 1997:715–822.
-
- Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res 1985;63:29–37. - PubMed
-
- Ayad M, Verity MA, Rubinstein EH. Lidocaine delays cortical ischemic depolarization: relationship to electrophysiologic recovery and neuropathology. J Neurosurg Anesthesiol 1994;6:98–110. - PubMed
-
- Somjen GG. Irreversible hypoxic (ischemic) neuron injury In: Somjen GG, ed. Ions in the brain. New York, NY: Oxford University Press, 2004:338–372.
-
- Norton L, Gibson RM, Gofton T, et al. Electroencephalographic recordings during withdrawal of life‐sustaining therapy until 30 minutes after declaration of death. Can J Neurol Sci 2017;44:139–145. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

