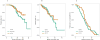Clinical Outcomes of First-line Abiraterone Acetate or Enzalutamide for Metastatic Castration-resistant Prostate Cancer After Androgen Deprivation Therapy + Docetaxel or ADT Alone for Metastatic Hormone-sensitive Prostate Cancer
- PMID: 29331381
- PMCID: PMC5986287
- DOI: 10.1016/j.clgc.2017.12.012
Clinical Outcomes of First-line Abiraterone Acetate or Enzalutamide for Metastatic Castration-resistant Prostate Cancer After Androgen Deprivation Therapy + Docetaxel or ADT Alone for Metastatic Hormone-sensitive Prostate Cancer
Abstract
Background: The CHAARTED (ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer) and STAMPEDE (Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy) trials showed that the addition of docetaxel (D) to androgen deprivation therapy (ADT) prolonged longevity of men with metastatic hormone-sensitive prostate cancer (mHSPC). However, the impact of upfront D on subsequent therapies is still unexplored. As abiraterone acetate (AA) and enzalutamide (E) are the most commonly used first-line treatment for metastatic castration-resistant prostate cancer (mCRPC), we aimed to assess whether they maintained their efficacy after ADT+D versus ADT alone.
Patients and methods: A cohort of patients with mCRPC treated between 2014 and 2017 with first-line AA or E for mCRPC was identified from 3 hospitals' institutional review board-approved databases. Patients were classified by use of D for mHSPC. This time frame was chosen as ADT+D became a valid therapeutic option for mHSPC in 2014, and it inherently entailed a short follow-up time on AA/E. The endpoints included overall survival from ADT start, overall survival from AA/E start, and time to AA/E start from ADT start. Differences between groups were assessed using the log-rank test.
Results: Of the 102 patients with mCRPC identified, 50 (49%) had previously received ADT alone, while 52 (51%) had ADT+D. No statistically significant difference in any of the evaluated outcomes was observed between the 2 cohorts. Yet, deaths in the ADT+D group were 12 versus 21 in the ADT alone, after a median follow-up of 24.4 and 29.8 months, respectively.
Conclusion: In a cohort of ADT/ADT+D-treated patients with mCRPC with short times to first-line AA/E and follow-up, the efficacy of AA/E is similar regardless of previous use of D.
Keywords: AR targeting agents; CHAARTED; mCRPC; mCRPC sequencing; mHSPC.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Philip W. Kantoff received compensation for scientific advisory board/consulting for Astellas, Bayer, Bellicum, BIND Biosciences, BN Immunotherapeutics, DRGT, Ipsen Pharmaceuticals, Janssen, Metamark, Merck, MTG Therapeutics, New England Research Institutes, Omnitura, OncoCellMDX, Progenity, Sanofi, Tarveda Therapeutics, Thermo Fisher Scientific; he has investment interests in Bellicum, DRGT, Metamark, Tarveda Therapeutics; and he is on data safety monitoring board of Genetech/Roche, Merck, Oncogenex. LCH reports compensation for consulting/advisory role at Dendreon, Genentech, KEW Group, Medivation/Astellas, Pfizer, Theragene; and institutional research funding from Bayer, Dendreon, Genentech/Roche, Medivation/Astellas, Sotio, Takeda, BMS; Travel, Accommodations, and Expenses from Sanofi. Mary-Ellen Taplin reports personal fees for attending advisory boards for Janssen and Medivation; and receives research funding from Janssen and Medivation. Daniel Y.C. Heng declares compensation for advisory board for Astellas and Janssen. Christopher J. Sweeney reports consulting with compensation for Astellas, Bayer, Genentech, Janssen, Pfizer, Sanofi; and received research funding from Astellas, Janssen, Sotio, Sanofi. Edoardo Francini declares that he has no conflict of interest, Steven Yip declares that he has no conflicts of interest, Shubidito Ahmed declares that he has no conflict of interest, Haocheng Li declares that he has no conflict of interest, Luke Ardolino declares that he has no conflict of interest, Carolyn P. Evan declares that she has no conflict of interest, Marina Kaymakcalan declares that she has no conflict of interest, Grace K. Shaw declares that she has no conflict of interest, Nimira S. Alimohamed declares that he has no conflict of interest, Anthony M. Joshua declares that he has no conflict of interest.
Figures
References
-
- Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer: II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43(2):209–223.
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. - PubMed
-
- Shiota M, Yokomizo A, Fujimoto N, Kuruma H, Naito S. Castration-resistant prostate cancer: novel therapeutics pre- or post-taxane administration. Curr Cancer Drug Targets. 2013;13:444–459. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials