Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis
- PMID: 29331880
- PMCID: PMC5936651
- DOI: 10.1016/j.jnutbio.2017.11.011
Preventive effects of indole-3-carbinol against alcohol-induced liver injury in mice via antioxidant, anti-inflammatory, and anti-apoptotic mechanisms: Role of gut-liver-adipose tissue axis
Abstract
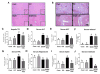
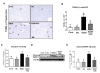
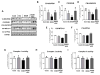
Indole-3-carbinol (I3C), found in Brassica family vegetables, exhibits antioxidant, anti-inflammatory, and anti-cancerous properties. Here, we aimed to evaluate the preventive effects of I3C against ethanol (EtOH)-induced liver injury and study the protective mechanism(s) by using the well-established chronic-plus-binge alcohol exposure model. The preventive effects of I3C were evaluated by conducting various histological, biochemical, and real-time PCR analyses in mouse liver, adipose tissue, and colon, since functional alterations of adipose tissue and intestine can also participate in promoting EtOH-induced liver damage. Daily treatment with I3C alleviated EtOH-induced liver injury and hepatocyte apoptosis, but not steatosis, by attenuating elevated oxidative stress, as evidenced by the decreased levels of hepatic lipid peroxidation, hydrogen peroxide, CYP2E1, NADPH-oxidase, and protein acetylation with maintenance of mitochondrial complex I, II, and III protein levels and activities. I3C also restored the hepatic antioxidant capacity by preventing EtOH-induced suppression of glutathione contents and mitochondrial aldehyde dehydrogenase-2 activity. I3C preventive effects were also achieved by attenuating the increased levels of hepatic proinflammatory cytokines, including IL1β, and neutrophil infiltration. I3C also attenuated EtOH-induced gut leakiness with decreased serum endotoxin levels through preventing EtOH-induced oxidative stress, apoptosis of enterocytes, and alteration of tight junction protein claudin-1. Furthermore, I3C alleviated adipose tissue inflammation and decreased free fatty acid release. Collectively, I3C prevented EtOH-induced liver injury via attenuating the damaging effect of ethanol on the gut-liver-adipose tissue axis. Therefore, I3C may also have a high potential for translational research in treating or preventing other types of hepatic injury associated with oxidative stress and inflammation.
Keywords: Alcohol; Apoptosis; Indole-3-carbinol; Inflammation; Liver; Oxidative stress.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures







References
-
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. - PubMed
-
- Lakshman MR. Some novel insights into the pathogenesis of alcoholic steatosis. Alcohol. 2004;34:45–8. - PubMed
-
- Natori S, Rust C, Stadheim LM, Srinivasan A, Burgart LJ, Gores GJ. Hepatocyte apoptosis is a pathologic feature of human alcoholic hepatitis. J Hepatol. 2001;34:248–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

