Effects of Macrolide and Corticosteroid in Neutrophilic Asthma Mouse Model
- PMID: 29332324
- PMCID: PMC5771750
- DOI: 10.4046/trd.2017.0108
Effects of Macrolide and Corticosteroid in Neutrophilic Asthma Mouse Model
Erratum in
-
Corrigendum: Effects of Macrolide and Corticosteroid in Neutrophilic Asthma Mouse Model.Tuberc Respir Dis (Seoul). 2018 Oct;81(4):350. doi: 10.4046/trd.2017.0108.r. Tuberc Respir Dis (Seoul). 2018. PMID: 30238718 Free PMC article.
Abstract
Background: Asthma is a disease of chronic airway inflammation with heterogeneous features. Neutrophilic asthma is corticosteroid-insensitive asthma related to absence or suppression of TH2 process and increased TH1 and/or TH17 process. Macrolides are immunomodulatory drug that reduce airway inflammation, but their role in asthma is not fully known. The purpose of this study was to evaluate the role of macrolides in neutrophilic asthma and compare their effects with those of corticosteroids.
Methods: C57BL/6 female mice were sensitized with ovalbumin (OVA) and lipopolysaccharides (LPS). Clarithromycin (CAM) and/or dexamethasone (DXM) were administered at days 14, 15, 21, 22, and 23. At day 24, the mice were sacrificed.
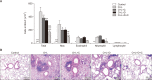
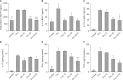
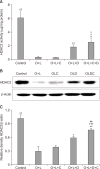
Results: Airway resistance in the OVA+LPS exposed mice was elevated but was more attenuated after treatment with CAM+DXM compared with the monotherapy group (p<0.05 and p<0.01). In bronchoalveolar lavage fluid study, total cells and neutrophil counts in OVA+LPS mice were elevated but decreased after CAM+DXM treatment. In hematoxylin and eosin stain, the CAM+DXM-treated group showed less inflammation additively than the monotherapy group. There was less total protein, interleukin 17 (IL-17), interferon γ, and tumor necrosis factor α in the CAM+DXM group than in the monotherapy group (p<0.001, p<0.05, and p<0.001). More histone deacetylase 2 (HDAC2) activity was recovered in the DXM and CAM+DXM challenged groups than in the control group (p<0.05).
Conclusion: Decreased IL-17 and recovered relative HDAC2 activity correlated with airway resistance and inflammation in a neutrophilic asthma mouse model. This result suggests macrolides as a potential corticosteroid-sparing agent in neutrophilic asthma.
Keywords: Adrenal Cortex Hormones; Asthma; Histone Deacetylases; Inflammation; Macrolides; Neutrophils; Th17 Cells.
Copyright©2018. The Korean Academy of Tuberculosis and Respiratory Diseases
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Reddel HK, Hurd SS, FitzGerald JM. World Asthma Day. GINA 2014: a global asthma strategy for a global problem. Int J Tuberc Lung Dis. 2014;18:505–506. - PubMed
-
- Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11:958–972. - PubMed
-
- Wenzel SE. Complex phenotypes in asthma: current definitions. Pulm Pharmacol Ther. 2013;26:710–715. - PubMed
-
- Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160:93–101. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

