Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing
- PMID: 29332973
- PMCID: PMC5759086
Metabologenomics of Phaeochromocytoma and Paraganglioma: An Integrated Approach for Personalised Biochemical and Genetic Testing
Abstract
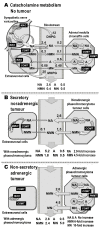
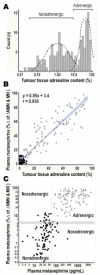
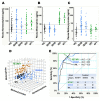
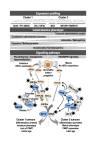
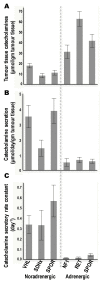
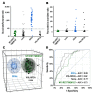
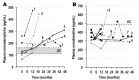
The tremendous advances over the past two decades in both clinical genetics and biochemical testing of chromaffin cell tumours have led to new considerations about how these aspects of laboratory medicine can be integrated to improve diagnosis and management of affected patients. With germline mutations in 15 genes now identified to be responsible for over a third of all cases of phaeochromocytomas and paragangliomas, these tumours are recognised to have one of the richest hereditary backgrounds among all neoplasms. Depending on the mutation, tumours show distinct differences in metabolic pathways that relate to or even directly impact clinical presentation. At the same time, there has been improved understanding about how catecholamines are synthesised, stored, secreted and metabolised by chromaffin cell tumours. Although the tumours may not always secrete catecholamines it has become clear that almost all continuously produce and metabolise catecholamines. This has not only fuelled changes in laboratory medicine, but has also assisted in recognition of genotype-biochemical phenotype relationships important for diagnostics and clinical care. In particular, differences in catecholamine and energy pathway metabolomes can guide genetic testing, assist with test interpretation and provide predictions about the nature, behaviour and imaging characteristics of the tumours. Conversely, results of genetic testing are important for guiding how routine biochemical testing should be employed and interpreted in surveillance programmes for at-risk patients. In these ways there are emerging needs for modern laboratory medicine to seamlessly integrate biochemical and genetic testing into the diagnosis and management of patients with chromaffin cell tumours.
Conflict of interest statement
Competing Interests: None declared.
Figures



References
-
- Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108–19. - PubMed
-
- Flynn A, Benn D, Clifton-Bligh R, Robinson B, Trainer AH, James P, et al. The genomic landscape of phaeochromocytoma. J Pathol. 2015;236:78–89. - PubMed
-
- Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11:101–11. - PubMed
-
- Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99:1915–42. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
