Ethical and Safety Issues of Stem Cell-Based Therapy
- PMID: 29333086
- PMCID: PMC5765738
- DOI: 10.7150/ijms.21666
Ethical and Safety Issues of Stem Cell-Based Therapy
Abstract
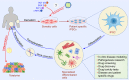
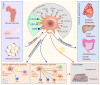
Results obtained from completed and on-going clinical studies indicate huge therapeutic potential of stem cell-based therapy in the treatment of degenerative, autoimmune and genetic disorders. However, clinical application of stem cells raises numerous ethical and safety concerns. In this review, we provide an overview of the most important ethical issues in stem cell therapy, as a contribution to the controversial debate about their clinical usage in regenerative and transplantation medicine. We describe ethical challenges regarding human embryonic stem cell (hESC) research, emphasizing that ethical dilemma involving the destruction of a human embryo is a major factor that may have limited the development of hESC-based clinical therapies. With previous derivation of induced pluripotent stem cells (iPSCs) this problem has been overcome, however current perspectives regarding clinical translation of iPSCs still remain. Unlimited differentiation potential of iPSCs which can be used in human reproductive cloning, as a risk for generation of genetically engineered human embryos and human-animal chimeras, is major ethical issue, while undesired differentiation and malignant transformation are major safety issues. Although clinical application of mesenchymal stem cells (MSCs) has shown beneficial effects in the therapy of autoimmune and chronic inflammatory diseases, the ability to promote tumor growth and metastasis and overestimated therapeutic potential of MSCs still provide concerns for the field of regenerative medicine. This review offers stem cell scientists, clinicians and patient's useful information and could be used as a starting point for more in-depth analysis of ethical and safety issues related to clinical application of stem cells.
Keywords: embryonic stem cells; induced pluripotent stem cells; mesenchymal stem cells; stem cell-based therapy..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Volarevic V, Ljujic B, Stojkovic P. et al. Human stem cell research and regenerative medicine: present and future. Br Med Bull. 2011;99:155–168. - PubMed
-
- Volarevic V, Erceg S, Bhattacharya SS. et al. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22:1309–1323. - PubMed
-
- Turner L, Knoepfler P. Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry. Cell Stem Cell. 2016;19:154–157. - PubMed
-
- Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. - PubMed
-
- Zhang X, Stojkovic P, Przyborski S. et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–2676. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

