Barriers to care for chronic hepatitis C in the direct-acting antiviral era: a single-centre experience
- PMID: 29333275
- PMCID: PMC5759739
- DOI: 10.1136/bmjgast-2017-000181
Barriers to care for chronic hepatitis C in the direct-acting antiviral era: a single-centre experience
Abstract
Background: Cure rates for chronic hepatitis C have improved dramatically with direct-acting antivirals (DAAs), but treatment barriers remain. We aimed to compare treatment initiation rates and barriers across both interferon-based and DAA-based eras.
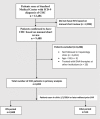
Methods: We conducted a retrospective cohort study of all patients with chronic hepatitis C seen at an academic hepatology clinic from 1999 to 2016. Patients were identified to have chronic hepatitis C by the International Classification of Diseases, Ninth Revision codes, and the diagnosis was validated by chart review. Patients were excluded if they did not have at least one visit in hepatology clinic, were under 18 years old or had prior treatment with DAA therapy. Patients were placed in the DAA group if they were seen after 1 January 2014 and had not yet achieved virological cure with prior treatment. All others were considered in the interferon group.
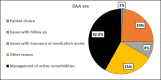
Results: 3202 patients were included (interferon era: n=2688; DAA era: n=514). Despite higher rates of decompensated cirrhosis and medical comorbidities in the DAA era, treatment and sustained virological response rates increased significantly when compared with the interferon era (76.7% vs 22.3%, P<0.001; 88.8% vs 55%, P<0.001, respectively). Lack of follow-up remained a significant reason for non-treatment in both groups (DAA era=24% and interferon era=45%). An additional 8% of patients in the DAA era were not treated due to insurance or issues with cost. In the DAA era, African-Americans, compared with Caucasians, had significantly lower odds of being treated (OR=0.37, P=0.02).
Conclusions: Despite higher rates of medical comorbidities in the DAA era, considerable treatment challenges remain including cost, loss to follow-up and ethnic disparities.
Keywords: chronic viral hepatitis; health service research; hepatitis c.
Conflict of interest statement
Competing interests: MHN: grant/research support: Bristol Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals and National Cancer Institute; advisory board/consultant: Anylam Pharmaceutical, Gilead Sciences, Dynax Laboratories and Intercept Pharmaceutical.
Figures

Similar articles
-
Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia.J Hepatol. 2019 Aug;71(2):281-288. doi: 10.1016/j.jhep.2019.04.014. Epub 2019 May 10. J Hepatol. 2019. PMID: 31078544
-
Effectiveness and cost of hepatitis C virus cryoglobulinaemia vasculitis treatment: From interferon-based to direct-acting antivirals era.Liver Int. 2017 Dec;37(12):1805-1813. doi: 10.1111/liv.13465. Epub 2017 May 29. Liver Int. 2017. PMID: 28467688
-
Identifying Barriers to the Treatment of Chronic Hepatitis C Infection.Dig Dis. 2020;38(1):46-52. doi: 10.1159/000501821. Epub 2019 Aug 16. Dig Dis. 2020. PMID: 31422405
-
Therapy of chronic hepatitis C virus infection in the era of direct-acting and host-targeting antiviral agents.J Infect. 2014 Jan;68(1):1-20. doi: 10.1016/j.jinf.2013.08.019. Epub 2013 Sep 4. J Infect. 2014. PMID: 24012819 Review.
-
Treatment of chronic HCV genotype 1 coinfection.Curr HIV/AIDS Rep. 2015 Sep;12(3):326-35. doi: 10.1007/s11904-015-0278-4. Curr HIV/AIDS Rep. 2015. PMID: 26228050 Review.
Cited by
-
Factors predicting staging and treatment initiation for patients with chronic hepatitis C infection: insurance a key predictor.J Public Health (Oxf). 2022 Mar 7;44(1):148-157. doi: 10.1093/pubmed/fdaa276. J Public Health (Oxf). 2022. PMID: 33539524 Free PMC article.
-
The Role of the Liver-Specific microRNA, miRNA-122 in the HCV Replication Cycle.Int J Mol Sci. 2020 Aug 7;21(16):5677. doi: 10.3390/ijms21165677. Int J Mol Sci. 2020. PMID: 32784807 Free PMC article. Review.
-
Characteristics and Treatment Rate of Patients With Hepatitis C Virus Infection in the Direct-Acting Antiviral Era and During the COVID-19 Pandemic in the United States.JAMA Netw Open. 2022 Dec 1;5(12):e2245424. doi: 10.1001/jamanetworkopen.2022.45424. JAMA Netw Open. 2022. PMID: 36477481 Free PMC article.
-
Clients' perceptions of barriers and facilitators to implementing hepatitis C virus care in homeless shelters.BMC Infect Dis. 2020 May 29;20(1):386. doi: 10.1186/s12879-020-05103-6. BMC Infect Dis. 2020. PMID: 32471376 Free PMC article.
-
Telemedicine successfully engages marginalized rural hepatitis C patients in curative care.J Assoc Med Microbiol Infect Dis Can. 2020 Jun 23;5(2):87-97. doi: 10.3138/jammi-2019-0025. eCollection 2020 Jun. J Assoc Med Microbiol Infect Dis Can. 2020. PMID: 36338186 Free PMC article.
References
-
- Butt AA, Justice AC, Skanderson M, et al. . Rate and predictors of treatment prescription for hepatitis C. Gut 2007;56:385–9. doi:10.1136/gut.2006.099150 - DOI - PMC - PubMed
-
- Feillant M, Jézéquel C, Lison H, et al. . Chronic hepatitis C: treat or wait? A prospective study on reasons for treatment or nontreatment in the era of first-generation protease inhibitors. Eur J Gastroenterol Hepatol 2016;28:164–72. doi:10.1097/MEG.0000000000000506 - DOI - PubMed
-
- Ghany MG, Strader DB, Thomas DL, et al. . Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335–74. doi:10.1002/hep.22759 - DOI - PMC - PubMed
-
- Kramer JR, Kanwal F, Richardson P, et al. . Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol 2012;56:320–5. doi:10.1016/j.jhep.2011.05.032 - DOI - PubMed
-
- Vutien P, Hoang J, Brooks L, et al. . Racial disparities in treatment rates for chronic hepatitis C: analysis of a population-based cohort of 73,665 patients in the United States. Medicine 2016;95:e3719 doi:10.1097/MD.0000000000003719 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
