Adiponectin inhibits inflammatory cytokines production by Beclin-1 phosphorylation and B-cell lymphoma 2 mRNA destabilization: role for autophagy induction
- PMID: 29333604
- PMCID: PMC5843704
- DOI: 10.1111/bph.14144
Adiponectin inhibits inflammatory cytokines production by Beclin-1 phosphorylation and B-cell lymphoma 2 mRNA destabilization: role for autophagy induction
Abstract
Background and purpose: Adiponectin potently suppresses inflammatory mediator production. Autophagy is known to play a critical role in the modulation of inflammatory responses by adiponectin. However, the underlying mechanisms are not clearly understood. Interaction between Beclin-1 and B-cell lymphoma 2 (Bcl-2) is a critical event in autophagy induction. We examined the effects of globular adiponectin (gAcrp) on the Beclin-1/Bcl-2 association and its underlying mechanisms.
Experimental approach: The effect of gAcrp on the interaction between Beclin-1 and Bcl-2 was examined by immunoprecipitation followed by Western blotting. To elucidate the underlying mechanisms, we determined the effects of gAcrp on Beclin-1 phosphorylation and Bcl-2 mRNA stability, and investigated their role in the suppression of inflammatory mediators using pharmacological inhibitors and transient target gene knockdown.
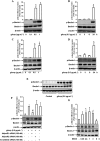
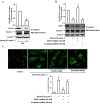
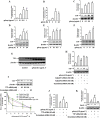
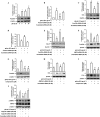
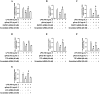
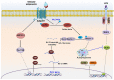
Key results: Globular adiponectin disrupted the association between Beclin-1 and Bcl-2 and increased Beclin-1 phosphorylation at Thr119 , critical residue for binding with Bcl-2, via a death-associated protein kinase-1 (DAPK1)-dependent mechanism. Moreover, gAcrp reduced Bcl-2 expression via Bcl-2 mRNA destabilization, without significantly affecting Bcl-2 promoter activity and protein degradation, which was mediated by tristetraprolin (TTP) induction. Finally, DAPK1 and TTP were shown to play key roles in gAcrp-induced autophagosome formation and suppression of LPS-stimulated TNF-α and IL-1β expression.
Conclusion and implications: Beclin-1 phosphorylation and Bcl-2 mRNA destabilization mediated by DAPK1 and TTP are crucial events leading to autophagy and the suppression of inflammatory cytokine production by gAcrp. These results provide novel mechanisms underlying adiponectin's modulation of inflammatory responses. DAPK and TTP are potential therapeutic targets for the management of inflammation.
© 2018 The British Pharmacological Society.
Figures


References
-
- Abrahamsen H, Stenmark H, Platta HW (2012). Ubiquitination and phosphorylation of Beclin 1 and its binding partners: tuning class III phosphatidylinositol 3‐kinase activity and tumor suppression. FEBS Lett 586: 1584–1591. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

