Ether-à-go-go K+ channels: effective modulators of neuronal excitability
- PMID: 29333676
- PMCID: PMC5830433
- DOI: 10.1113/JP275477
Ether-à-go-go K+ channels: effective modulators of neuronal excitability
Abstract
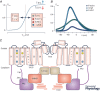
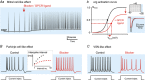
Mammalian ether-à-go-go (EAG) channels are voltage-gated K+ channels. They are encoded by the KCNH gene family and divided into three subfamilies, eag (Kv10), erg (eag-related gene; Kv11) and elk (eag-like; Kv12). All EAG channel subtypes are expressed in the brain where they effectively modulate neuronal excitability. This Topical Review describes the biophysical properties of each of the EAG channel subtypes, their function in neurons and the neurological diseases induced by EAG channel mutations. In contrast to the function of erg currents in the heart, where they contribute to repolarization of the cardiac action potential, erg currents in neurons are involved in the maintenance of the resting potential, setting of action potential threshold and frequency accommodation. They can even support high frequency firing by preventing a depolarization-induced Na+ channel block. EAG channels are modulated differentially, e.g. eag channels by intracellular Ca2+ , erg channels by extracellular K+ and GPCRs, and elk channels by changes in pH. So far, only currents mediated by erg channels have been recorded in neurons with the help of selective blockers. Neuronal eag and elk currents have not been isolated due to the lack of suitable channel blockers. However, findings in KO mice indicate a physiological role of eag1 currents in synaptic transmission and an involvement of elk2 currents in cognitive performance. Human eag1 and eag2 gain-of-function mutations underlie syndromes associated with epileptic seizures.
Keywords: Ether-à-go-go K+ channel; HERG; Potassium channel; neuronal excitability.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures

References
-
- Alonso‐Ron C, Barros F, Manso DG, Gomez‐Varela D, Miranda P, Carretero L, Dominguez P & de la Pena P (2009). Participation of HERG channel cytoplasmic structures on regulation by the G protein‐coupled TRH receptor. Pflugers Arch 457, 1237–1252. - PubMed
-
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S & Becchetti A (2009). Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem 16, 66–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

