Pharmacogenetic analysis of opioid dependence treatment dose and dropout rate
- PMID: 29333880
- PMCID: PMC5940523
- DOI: 10.1080/00952990.2017.1420795
Pharmacogenetic analysis of opioid dependence treatment dose and dropout rate
Abstract
Background: Currently, no pharmacogenetic tests for selecting an opioid-dependence pharmacotherapy have been approved by the US Food and Drug Administration.
Objectives: Determine the effects of variants in 11 genes on dropout rate and dose in patients receiving methadone or buprenorphine/naloxone (ClinicalTrials.gov Identifier: NCT00315341).
Methods: Variants in six pharmacokinetic genes (CYP1A2, CYP2B6, CYP2C19, CYP2C9, CYP2D6, CYP3A4) and five pharmacodynamic genes (HTR2A, OPRM1, ADRA2A, COMT, SLC6A4) were genotyped in samples from a 24-week, randomized, open-label trial of methadone and buprenorphine/naloxone for the treatment of opioid dependence (n = 764; 68.7% male). Genotypes were then used to determine the metabolism phenotype for each pharmacokinetic gene. Phenotypes or genotypes for each gene were analyzed for association with dropout rate and mean dose.
Results: Genotype for 5-HTTLPR in the SLC6A4 gene was nominally associated with dropout rate when the methadone and buprenorphine/naloxone groups were combined. When the most significant variants associated with dropout rate were analyzed using pairwise analyses, SLC6A4 (5-HTTLPR) and COMT (Val158Met; rs4860) had nominally significant associations with dropout rate in methadone patients. None of the genes analyzed in the study was associated with mean dose of methadone or buprenorphine/naloxone.
Conclusions: This study suggests that functional polymorphisms related to synaptic dopamine or serotonin levels may predict dropout rates during methadone treatment. Patients with the S/S genotype at 5-HTTLPR in SLC6A4 or the Val/Val genotype at Val158Met in COMT may require additional treatment to improve their chances of completing addiction treatment. Replication in other methadone patient populations will be necessary to ensure the validity of these findings.
Keywords: COMT; Opioid dependence; SERT; buprenorphine; methadone; pharmacogenetics.
Conflict of interest statement
Genotyping on Assurex Health GeneSight® Analgesic, Psychotropic and ADHD panels was performed by Assurex Health Inc. as an in-kind donation. J.L., A.G., and B.M.D. are employees and shareholders of Assurex Health Inc. The authors declare no other conflicts of interest.
Figures
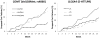
References
-
- Brewer DD, Catalano RF, Haggerty K, Gainey RR, Fleming CB. A meta-analysis of predictors of continued drug use during and after treatment for opiate addiction. Addiction. 1998 Jan;93(1):73–92. - PubMed
-
- Senbanjo R, Wolff K, Marshall EJ, Strang J. Persistence of heroin use despite methadone treatment: poor coping self-efficacy predicts continued heroin use. Drug Alcohol Rev. 2009 Nov;28(6):608–15. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
