Exogenous oestrogen inhibits genital transmission of cell-associated HIV-1 in DMPA-treated humanized mice
- PMID: 29334191
- PMCID: PMC5810324
- DOI: 10.1002/jia2.25063
Exogenous oestrogen inhibits genital transmission of cell-associated HIV-1 in DMPA-treated humanized mice
Abstract
Introduction: HIV affects more women than any other life-threatening infectious agent, and most infections are sexually transmitted. HIV must breach the female genital tract mucosal barrier to establish systemic infection, and clinical studies indicate virus more easily evades this barrier in women using depot-medroxyprogesterone acetate (DMPA) and other injectable progestins for contraception. Identifying a potential mechanism for this association, we learned DMPA promotes susceptibility of wild-type mice to genital herpes simplex virus type 2 (HSV-2) infection by reducing genital tissue expression of the cell-cell adhesion molecule desmoglein-1 (DSG-1) and increasing genital mucosal permeability. Conversely, DMPA-mediated increases in genital mucosal permeability and HSV-2 susceptibility were eliminated in mice concomitantly administered exogenous oestrogen (E). To confirm and extend these findings, herein we used humanized mice to define effects of systemic DMPA and intravaginal (ivag) E administration on susceptibility to genital infection with cell-associated HIV-1.
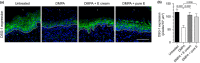
Methods: Effects of DMPA or an intravaginal (ivag) E cream on engraftment of NOD-scid-IL-2Rgcnull (NSG) mice with human peripheral blood mononuclear cells (hPBMCs) were defined with flow cytometry. Confocal microscopy was used to evaluate effects of DMPA, DMPA and E cream, or DMPA and the pharmacologically active component of the cream on vaginal tissue DSG-1 expression and genital mucosal permeability to low molecular weight (LMW) molecules and hPBMCs. In other studies, hPBMC-engrafted NSG mice (hPBMC-NSG) received DMPA or DMPA and ivag E cream before genital inoculation with 106 HIV-1-infected hPBMCs. Mice were euthanized 10 days after infection, and plasma HIV-1 load quantified by qRT-PCR and splenocytes used to detect HIV-1 p24 antigen via immunohistochemistry and infectious virus via TZM-bl luciferase assay.
Results: Whereas hPBMC engraftment was unaffected by DMPA or E treatment, mice administered DMPA and E (cream or the pharmacologically active cream component) displayed greater vaginal tissue expression of DSG-1 protein and decreased vaginal mucosal permeability to LMW molecules and hPBMCs versus DMPA-treated mice. DMPA-treated hPBMC-NSG mice were also uniformly susceptible to genital transmission of cell-associated HIV-1, while no animal concomitantly administered DMPA and E cream acquired systemic HIV-1 infection.
Conclusion: Exogenous E administration reduces susceptibility of DMPA-treated humanized mice to genital HIV-1 infection.
Keywords: DMPA; HIV prevention; genital HIV transmission; humanized mice; oestrogen.
© 2018 The Authors. Journal of the International AIDS Society published by John Wiley & sons Ltd on behalf of the International AIDS Society.
Figures



References
-
- HIV/AIDS JUNPo . Global AIDS Update 2016. UNAIDS Switzerland. 2017. [cited 2017 June 1]. Available from: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016.
-
- Leclerc‐Madlala S. Age‐disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008;22(Suppl 4):S17–25. - PubMed
-
- Polis CB, Phillips SJ, Curtis KM, Westreich DJ, Steyn PS, Raymond E, et al. Hormonal contraceptive methods and risk of HIV acquisition in women: a systematic review of epidemiological evidence. Contraception. 2014;90(4):360–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

