Dendritic polyglycerol nanoparticles show charge dependent bio-distribution in early human placental explants and reduce hCG secretion
- PMID: 29334310
- PMCID: PMC5815307
- DOI: 10.1080/17435390.2018.1425496
Dendritic polyglycerol nanoparticles show charge dependent bio-distribution in early human placental explants and reduce hCG secretion
Abstract
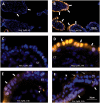
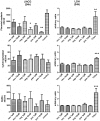
A thorough understanding of nanoparticle bio-distribution at the feto-maternal interface will be a prerequisite for their diagnostic or therapeutic application in women of childbearing age and for teratologic risk assessment. Therefore, the tissue interaction of biocompatible dendritic polyglycerol nanoparticles (dPG-NPs) with first- trimester human placental explants were analyzed and compared to less sophisticated trophoblast-cell based models. First-trimester human placental explants, BeWo cells and primary trophoblast cells from human term placenta were exposed to fluorescence labeled, ∼5 nm dPG-NPs, with differently charged surfaces, at concentrations of 1 µM and 10 nM, for 6 and 24 h. Accumulation of dPGs was visualized by fluorescence microscopy. To assess the impact of dPG-NP on trophoblast integrity and endocrine function, LDH, and hCG releases were measured. A dose- and charge-dependent accumulation of dPG-NPs was observed at the early placental barrier and in cell lines, with positive dPG-NP-surface causing deposits even in the mesenchymal core of the placental villi. No signs of plasma membrane damage could be detected. After 24 h we observed a significant reduction of hCG secretion in placental explants, without significant changes in trophoblast apoptosis, at low concentrations of charged dPG-NPs. In conclusion, dPG-NP's surface charge substantially influences their bio-distribution at the feto-maternal interface, with positive charge facilitating trans-trophoblast passage, and in contrast to more artificial models, the first-trimester placental explant culture model reveals potentially hazardous influences of charged dPG-NPs on early placental physiology.
Keywords: BeWo; Dendritic polyglycerol nanoparticles; early human placenta; hCG; nanotoxicology; primary trophoblasts.
Figures


Similar articles
-
Purified first and third trimester placental trophoblasts differ in in vitro hormone secretion.J Clin Endocrinol Metab. 1990 Apr;70(4):1187-92. doi: 10.1210/jcem-70-4-1187. J Clin Endocrinol Metab. 1990. PMID: 2318939
-
Dynamic changes in hyperglycosylated human chorionic gonadotrophin throughout the first trimester of pregnancy and its role in early placentation.Hum Reprod. 2015 May;30(5):1029-38. doi: 10.1093/humrep/dev016. Epub 2015 Mar 4. Hum Reprod. 2015. PMID: 25743784
-
Gestational age-dependent dual action of epidermal growth factor on human placenta early in gestation.J Clin Endocrinol Metab. 1992 Nov;75(5):1362-7. doi: 10.1210/jcem.75.5.1430098. J Clin Endocrinol Metab. 1992. PMID: 1430098
-
Human chorionic gonadotropin: Different glycoforms and biological activity depending on its source of production.Ann Endocrinol (Paris). 2016 Jun;77(2):75-81. doi: 10.1016/j.ando.2016.04.012. Epub 2016 May 10. Ann Endocrinol (Paris). 2016. PMID: 27177499 Review.
-
Maternal platelets at the first trimester maternal-placental interface - Small players with great impact on placenta development.Placenta. 2022 Jul;125:61-67. doi: 10.1016/j.placenta.2021.12.009. Epub 2021 Dec 9. Placenta. 2022. PMID: 34920861 Review.
Cited by
-
Recent insights on indirect mechanisms in developmental toxicity of nanomaterials.Part Fibre Toxicol. 2020 Jul 11;17(1):31. doi: 10.1186/s12989-020-00359-x. Part Fibre Toxicol. 2020. PMID: 32653006 Free PMC article. Review.
-
Rational Design of Nanomedicine for Placental Disorders: Birthing a New Era in Women's Reproductive Health.Small. 2024 Oct;20(41):e2300852. doi: 10.1002/smll.202300852. Epub 2023 May 16. Small. 2024. PMID: 37191231 Review.
-
Placental Models for Evaluation of Nanocarriers as Drug Delivery Systems for Pregnancy Associated Disorders.Biomedicines. 2022 Apr 19;10(5):936. doi: 10.3390/biomedicines10050936. Biomedicines. 2022. PMID: 35625672 Free PMC article. Review.
-
Uptake and transport of pullulan acetate nanoparticles in the BeWo b30 placental barrier cell model.Int J Nanomedicine. 2018 Jul 11;13:4073-4082. doi: 10.2147/IJN.S161319. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30034233 Free PMC article.
-
Size-Dependent Cytotoxicity of Hydroxyapatite Crystals on Renal Epithelial Cells.Int J Nanomedicine. 2020 Jul 15;15:5043-5060. doi: 10.2147/IJN.S232926. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32764935 Free PMC article.
References
-
- Ali H., Kalashnikova I., White M. A., Sherman M., and Rytting E.. 2013. “Preparation, Characterization, and Transport of Dexamethasone-Loaded Polymeric Nanoparticles across a Human Placental in Vitro Model.” International Journal of Pharmaceutics 454 (1): 149–157. doi:10.1016/j.ijpharm.2013.07.010. - DOI - PMC - PubMed
-
- Barnea E. R., Shurtz-Swirski R., and Kaplan M.. 1992. “Factors Controlling Spontaneous Human Chorionic Gonadotrophin in Superfused First Trimester Placental Explants.” Human Reproduction (Oxford, England) 7 (7): 1022–1026. - PubMed
-
- Belisle S., Petit A., Gallo-Payet N., Lehoux J., Bellabarba D., Escher E., and Guillon G.. 1992. “Endocrine Control of hPL and hCG Production by the Human Placenta.” Placenta 13: 163–172. - PubMed
-
- Benirschke K., and Kaufmann P.. 2012. Pathology of the Human Placenta. 6th ed New York, NY: Springer.
-
- Bourne H. R., and von Zastrow M.. 2001. Drug receptors and pharmacodynamics In Basic and Clinical Pharmacology, edited by Katzung B. G., 8th ed New York, NY: McGraw-Hill.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
