Pot-Economy Autooxidative Condensation of 2-Aryl-2-lithio-1,3-dithianes
- PMID: 29334462
- PMCID: PMC6150673
- DOI: 10.1021/acs.joc.7b02896
Pot-Economy Autooxidative Condensation of 2-Aryl-2-lithio-1,3-dithianes
Abstract
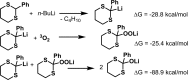
The autoxidative condensation of 2-aryl-2-lithio-1,3-dithianes is here reported. Treatment of 2-aryl-1,3-dithianes with n-BuLi in the absence of any electrophile leads to condensation of three molecules of 1,3-dithianes and formation of highly functionalized α-thioether ketones orthothioesters in 51-89% yields upon air exposure. The method was further expanded to benzaldehyde dithioacetals, affording corresponding orthothioesters and α-thioether ketones in 48-97% yields. The experimental results combined with density functional theory studies support a mechanism triggered by the autoxidation of 2-aryl-2-lithio-1,3-dithianes to yield a highly reactive thioester that undergoes condensation with two other molecules of 2-aryl-2-lithio-1,3-dithiane.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Boche G.; Lohrenz J. C. W. Chem. Rev. 2001, 101, 697–756. 10.1021/cr940260x. - DOI - PubMed
- Wardell J. L. In Comprehensive Organometallic Chemistry; Stone F. G. A., Abel E. W., Eds.; Pergamon: Oxford, 1982; pp 43–120.
- Sosnovsky G.; Brown J. H. Chem. Rev. 1966, 66, 529–566. 10.1021/cr60243a003. - DOI - PubMed
-
- Müller E.; Töpel T. Ber. Dtsch. Chem. Ges. B 1939, 72, 273–290. 10.1002/cber.19390720208. - DOI
-
- Jones A. B.; Wang J.; Hamme A. T.; Han W.. Oxygen. In Encyclopedia of Reagents for Organic Synthesis; John Wiley & Sons, 2001.
- Boche G.; Möbus K.; Harms K.; Lohrenz J. C. W.; Marsch M. Chem. - Eur. J. 1996, 2, 604–607. 10.1002/chem.19960020521. - DOI - PubMed
- Julia M.; Saint-Jalmes V. P.; Verpeaux J.-N. Synlett 1993, 1993, 233–234. 10.1055/s-1993-22415. - DOI
- Boche G.; Bosold F.; Lohrenz J. C. W. Angew. Chem., Int. Ed. Engl. 1994, 33, 1161–1163. 10.1002/anie.199411611. - DOI
-
-
Examples on the use of oxidation of organolithiums with O2:
- Möller M.; Husemann M.; Boche G. J. Organomet. Chem. 2001, 624, 47–52. 10.1016/S0022-328X(00)00596-9. - DOI
- Weber B. Synthesis 1999, 1999, 1593–1606. 10.1055/s-1999-3576. - DOI
- Barluenga J.; Foubelo F.; Fañanás F. J.; Yus M. Tetrahedron 1989, 45, 2183–2192. 10.1016/S0040-4020(01)80078-8. - DOI
- Ryckman D. M.; Stevens R. V. J. Am. Chem. Soc. 1987, 109, 4940–4948. 10.1021/ja00250a030. - DOI
- Hoell D.; Schnieders C.; Müllen K. Angew. Chem., Int. Ed. Engl. 1983, 22, 243–244. 10.1002/anie.198302431. - DOI
- Nguyen T. H.; Chau N. T.; Castanet A. S.; Nguyen K. P.; Mortier J. J. Org. Chem. 2007, 72, 3419–3429. 10.1021/jo070082a. - DOI - PubMed
- Einhorn J.; Luche J.-L.; Demerseman P. J. Chem. Soc., Chem. Commun. 1988, 1350. 10.1039/c39880001350. - DOI
- Parker K. A.; Koziski K. A. J. Org. Chem. 1987, 52, 674–676. 10.1021/jo00380a034. - DOI
-
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

