Blood biomarkers of expressed and inducible HIV-1
- PMID: 29334544
- PMCID: PMC5854535
- DOI: 10.1097/QAD.0000000000001748
Blood biomarkers of expressed and inducible HIV-1
Abstract
Objective: To define the relationships between molecular measures of viral persistence in blood (i.e., plasma viremia, cellular HIV-1 DNA, and mRNA) and expressed or inducible virus from resting CD4 T cells of individuals on suppressive antiretroviral therapy.
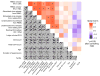
Design: We compared molecular measurements of HIV-1 in plasma and in uncultured peripheral blood mononuclear cells (PBMCs) to the levels of virions produced by either unstimulated or phorbol myristate acetate and ionomycin (PMA/iono)-stimulated PBMC or resting CD4 T cells from 21 donors on suppressive antiretroviral therapy.
Results: We found that unstimulated virion release from cultured resting CD4 T cells was positively correlated with the levels of plasma viremia in vivo (Spearman rho = 0.67, P = 0.0017). We also found that levels of both cellular HIV-1 DNA and unspliced HIV-1 mRNA per million uncultured PBMC were positively correlated with the levels of inducible virion release from both PMA/iono-stimulated PBMC (total HIV-1 DNA: rho = 0.64, P = 0.0017; unspliced HIV-1 RNA: rho = 0.77, P < 0.001) and PMA/iono-stimulated resting CD4 T cells (total HIV-1 DNA: rho = 0.75, P < 0.001; unspliced HIV-1 RNA: rho = 0.75, P < 0.001).
Conclusion: These results show for the first time that there are strong associations between in-vivo measures of HIV-1 persistence and ex-vivo measures of spontaneous and inducible virus production from cultured PBMC and resting CD4 T cells. Findings from this study provide insight into the biology of HIV-1 persistence and suggest methods to guide the evaluation of clinical strategies to reduce the size of the viral reservoir.
Conflict of interest statement
Figures




References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with Indinavir, Zidovudine, and Lamivudine in Adults with Human Immunodeficiency Virus Infection and Prior Antiretroviral Therapy. New England Journal of Medicine. 1997;337(11):734–739. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733. - PubMed
-
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, Emtricitabine, and Efavirenz vs. Zidovudine, Lamivudine, and Efavirenz for HIV. New England Journal of Medicine. 2006;354(3):251–260. - PubMed
-
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, et al. Dolutegravir plus Abacavir–Lamivudine for the Treatment of HIV-1 Infection. New England Journal of Medicine. 2013;369(19):1807–1818. - PubMed
-
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582–1586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

