Phase II Study of Dovitinib in Patients with Castration-Resistant Prostate Cancer (KCSG-GU11-05)
- PMID: 29334610
- PMCID: PMC6192917
- DOI: 10.4143/crt.2017.438
Phase II Study of Dovitinib in Patients with Castration-Resistant Prostate Cancer (KCSG-GU11-05)
Abstract
Purpose: Fibroblast growth factor (FGF) signals are important in carcinogenesis and progression of prostate cancer. Dovitinib is an oral, pan-class inhibitor of vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor, and fibroblast growth factor receptor (FGFR). We evaluated the efficacy and toxicity of dovitinib in men with metastatic castration resistant prostate cancer (mCRPC).
Materials and methods: This study was a single-arm, phase II, open-label, multicenter trial of dovitinib 500 mg/day (5-days-on/2-days-off schedule). The primary endpointwas 16-week progression-free survival (PFS). Secondary endpoints were overall survival (OS), toxicity and prostate-specific antigen (PSA) response rate. Biomarker analyses for VEGFR2, FGF23, and FGFR2 using multiplex enzyme-linked immunosorbent assay was performed.
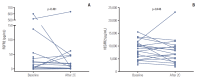
Results: Forty-four men were accrued from 11 hospitals. Eighty percent were post-docetaxel. Median PSA was 100 ng/dL, median age was 69, 82% had bone metastases, and 23% had liver metastases. Median cycles of dovitinibwas 2 (range, 0 to 33). Median PFSwas 3.67 months (95% confidence interval [CI], 1.36 to 5.98) and median OS was 13.70 months (95% CI, 0 to 27.41). Chemotherapy-naïve patients had longer PFS (17.90 months; 95% CI, 9.23 to 28.57) compared with docetaxel-treated patients (2.07 months; 95% CI, 1.73 to 2.41; p=0.001) and the patients with high serum VEGFR2 level over median level (7,800 pg/mL) showed longer PFS compared with others (6.03 months [95% CI, 4.26 to 7.80] vs. 1.97 months [95% CI, 1.79 to 2.15], p=0.023). Grade 3 related adverse events were seen in 40.9% of patients. Grade 1-2 nausea, diarrhea, fatigue, anorexia, and all grade thrombocytopenia are common.
Conclusion: Dovitinib showed modest antitumor activity with manageable toxicities in men with mCRPC. Especially, patients who were chemo-naïve benefitted from dovitinib.
Keywords: Biomarkers; Castration-Resistant Prostatic Neoplasm; Dovitinib.
Conflict of interest statement
We thank Novartis for their kind donation of dovitinib for this study.
Figures



Similar articles
-
Phase I/II and pharmacodynamic study of dovitinib (TKI258), an inhibitor of fibroblast growth factor receptors and VEGF receptors, in patients with advanced melanoma.Clin Cancer Res. 2011 Dec 1;17(23):7451-61. doi: 10.1158/1078-0432.CCR-11-1747. Epub 2011 Oct 5. Clin Cancer Res. 2011. PMID: 21976540 Clinical Trial.
-
An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer.Eur J Cancer. 2015 Aug;51(12):1588-95. doi: 10.1016/j.ejca.2015.05.020. Epub 2015 Jun 9. Eur J Cancer. 2015. PMID: 26070683 Clinical Trial.
-
Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial.Eur Urol. 2018 May;73(5):696-703. doi: 10.1016/j.eururo.2017.09.022. Epub 2017 Oct 23. Eur Urol. 2018. PMID: 29074061 Clinical Trial.
-
Analysis of serum protein biomarkers, circulating tumor DNA, and dovitinib activity in patients with tyrosine kinase inhibitor-refractory gastrointestinal stromal tumors.Ann Oncol. 2014 Nov;25(11):2272-2277. doi: 10.1093/annonc/mdu386. Epub 2014 Aug 22. Ann Oncol. 2014. PMID: 25149706 Clinical Trial.
-
Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma.Hepatology. 2016 Sep;64(3):774-84. doi: 10.1002/hep.28600. Epub 2016 May 17. Hepatology. 2016. PMID: 27082062 Clinical Trial.
Cited by
-
Potential Role of Targeting KDR and Proteasome Inhibitors in the Therapy of Esophageal Squamous Cell Carcinoma.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820948060. doi: 10.1177/1533033820948060. Technol Cancer Res Treat. 2020. PMID: 32924793 Free PMC article.
-
A Novel FGFR3 Splice Variant Preferentially Expressed in African American Prostate Cancer Drives Aggressive Phenotypes and Docetaxel Resistance.Mol Cancer Res. 2019 Oct;17(10):2115-2125. doi: 10.1158/1541-7786.MCR-19-0415. Epub 2019 Jul 2. Mol Cancer Res. 2019. PMID: 31266816 Free PMC article.
-
Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value.Cells. 2023 Mar 24;12(7):1002. doi: 10.3390/cells12071002. Cells. 2023. PMID: 37048074 Free PMC article. Review.
-
Neoadjuvant Treatment with Angiogenesis-Inhibitor Dovitinib Prior to Local Therapy in Hepatocellular Carcinoma: A Phase II Study.Oncologist. 2021 Oct;26(10):854-864. doi: 10.1002/onco.13901. Epub 2021 Aug 4. Oncologist. 2021. PMID: 34251745 Free PMC article. Clinical Trial.
-
FGFR families: biological functions and therapeutic interventions in tumors.MedComm (2020). 2023 Sep 23;4(5):e367. doi: 10.1002/mco2.367. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37750089 Free PMC article. Review.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Heer R, Douglas D, Mathers ME, Robson CN, Leung HY. Fibroblast growth factor 17 is over-expressed in human prostate cancer. J Pathol. 2004;204:578–86. - PubMed
-
- Giri D, Ropiquet F, Ittmann M. Alterations in expression of basic fibroblast growth factor (FGF) 2 and its receptor FGFR-1 in human prostate cancer. Clin Cancer Res. 1999;5:1063–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

